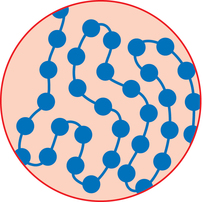
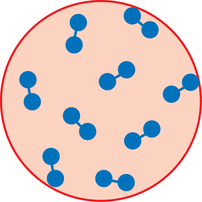
1. Explain why certain medications must be sterile. 2. Discuss the history of aseptic preparation and the organizations that provide guidelines. 3. Define the term aseptic technique, and demonstrate proper handwashing procedures. 4. Discuss the responsibilities, regulations, and workplace settings of personnel who compound parenteral preparations. Condition free from germs, infection, or any form of life Centers for Disease Control (CDC) Compounded sterile preparations (CSP) Medications prepared using sterile technique Bacteria that resides on the skin’s outer surface but does not cause disease An organism (such as a bacterium, virus, or protozoan) of microscopic size Any medication route other than the alimentary canal (digestion system) CDC guidelines that promote hand hygiene and the use of personal protective equipment (PPE) Free of living organisms, especially microorganisms United States Pharmacopoeia (USP) Pharmacy technicians must have a good understanding of aseptic technique and the practices surrounding the preparation of sterile products to ensure safety, accuracy, and correctness of the medication. In this chapter we will discuss the history and concept of asepsis, along with the regulations and responsibilities of personnel who prepare intravenous admixtures (Figure 1-1). We will also discuss handwashing and the correct performance procedure according to the Centers for Disease Control (CDC) guidelines. Following the proper procedures is the only way to ensure that contamination does not occur when performing aseptic technique. Patients receiving intravenous medications are often hospitalized and immune compromised, and they are more susceptible to infection (Figure 1-2). Since sterile intravenous products can be prepared in many settings, such as hospitals, outpatient pharmacies and clinics, doctor’s offices, and in a patient’s home, the need for extremely clean or sterile preparation is imperative to ensure the wellbeing of these patients. Typically, the normal bacteria that we all have on the surfaces of our body do not affect us because we are healthy, but to a sick person these can cause significant harm, and since patients receiving intravenous therapy are usually the most critical, every precaution must be taken to avoid contamination. The personnel responsible for their preparation must follow certain rules and guidelines as well as use specialized equipment. Technicians perform most of the preparation duties today under the direct supervision of a pharmacist. Louis Pasteur’s germ theory in the early 1800’s specified that bacteria caused diseases (Figure 1-3). Practices that are common today, such as handwashing, were not practiced routinely. As a result, many deaths were attributed to the unclean conditions of operating rooms and personnel practices. A person was more likely to die of postoperative gangrene than the surgery itself. As a result, many changes in the practices were established. In 1865, Sir Joseph Lister, a well-known surgeon, read a paper by Louis Pasteur and learned about the germ theory of disease. He stated that if infections were caused by microbes, the best way to prevent infections would be to kill the microbes before they reached the open wound. Lister used carbolic acid to kill germs. He wrote about the use of this acid in his work, Antiseptic Principle of the Surgery Practice. (essortment.com) The use of sanitary dressings and instruments led to the development of disposable supplies, such as syringes, needles, and other intravenous supplies in the 1920’s (Figure 1-4). Sterile solutions and equipment became accepted in health care in the 1930’s. In the 1960’s, following a rash of serious patient complications, the National Coordinating Committee on Large Volume Parenterals (NCCLVP) published the first set of recommendations for pharmacy and other health care professionals. In 1972, the Baxter Corporation produced a training manual which was later revised in 1990. Following these guidelines, American Society of Health-System Pharmacists (ASHP) and United States Pharmacopoeia (USP) published updated guidelines that are considered standard practices for pharmacy personnel when preparing sterile preparations or compounded sterile preparations (CSPs). Bacteria are stained with a substance called crystal violet. Those that retain the color are gram positive, and those that lose the color are gram negative. Antibiotic drugs are grouped together or classified based on their activity against gram positive organisms, such as staphylococci (Figure 1-5), or against gram negative organisms, such as aminoglycosides activity against diplococci (Figure 1-6). Once the bacteria is determined to be gram positive or gram negative, a physician can prescribe the medication that works most effectively for either a gram positive or a gram negative bacteria. • Policy and procedures for compounding • Provision of records or documentation as part of an ongoing quality assurance program • Proper training of pharmacists and technicians in aseptic technique • Provision of reference materials • Separate facilities and air quality controls to ensure that sterile conditions are available for aseptic compounding • Establishment of storage and beyond use dates for sterile products
The basics of aseptic preparations
Introduction

History of aseptic preparations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The basics of aseptic preparations