(IFN-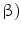 induction. The results showed that inflammatory signals can be detected by in vivo imaging after subcutaneous implantation of biocompatible or immune stimulatory implants. However, there were specific differences depending upon the assay system. The response to inflammatory proteases and cell growth signaling molecules appeared delocalized and was difficult to assign to one of several implants in individual animals. On the other hand, the interferon response was locally focused and was highly specific for pathogens whereas no signal was detected in response to wounding or to biocompatible implant materials. In conclusion, of the various detection systems investigated, the transgenic interferon mouse model could be applied to monitor bacterial implant infections and will be useful to evaluate the efficacy of antimicrobial implant coatings.
induction. The results showed that inflammatory signals can be detected by in vivo imaging after subcutaneous implantation of biocompatible or immune stimulatory implants. However, there were specific differences depending upon the assay system. The response to inflammatory proteases and cell growth signaling molecules appeared delocalized and was difficult to assign to one of several implants in individual animals. On the other hand, the interferon response was locally focused and was highly specific for pathogens whereas no signal was detected in response to wounding or to biocompatible implant materials. In conclusion, of the various detection systems investigated, the transgenic interferon mouse model could be applied to monitor bacterial implant infections and will be useful to evaluate the efficacy of antimicrobial implant coatings.
1 Introduction
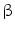 (IFN-
(IFN-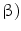 . This has originally been discovered as part of the antiviral response but it is also induced by diverse bacteria [30, 31]. Bacterial infections could be visualized by using a transgenic mouse model in which the interferon(IFN)-
. This has originally been discovered as part of the antiviral response but it is also induced by diverse bacteria [30, 31]. Bacterial infections could be visualized by using a transgenic mouse model in which the interferon(IFN)-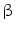 gene is replaced by a luciferase reporter gene that can be used for imaging purposes [32]. For imaging, heterozygous mice were used to allow IFN-
gene is replaced by a luciferase reporter gene that can be used for imaging purposes [32]. For imaging, heterozygous mice were used to allow IFN-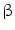 production from the wild type allele. The suitability of these in vivo imaging approaches was compared for the reliable evaluation and ranking of the biocompatibility of various implant materials and for detecting bacterial infections events in real time, respectively.
production from the wild type allele. The suitability of these in vivo imaging approaches was compared for the reliable evaluation and ranking of the biocompatibility of various implant materials and for detecting bacterial infections events in real time, respectively.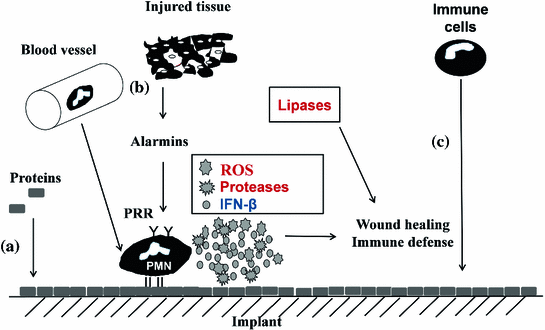
2 Materials and Methods
2.1 Preparation of Hydrocyanine
 , Germany). 3 mg of sodium borohydride (Sigma Aldrich) was added to carry out reduction reaction. The solution was stirred continuously for 5 mins in absence of oxygen. Solvent was evaporated in the presence of vacuum using rotary evaporators. The dried powder was stored at
, Germany). 3 mg of sodium borohydride (Sigma Aldrich) was added to carry out reduction reaction. The solution was stirred continuously for 5 mins in absence of oxygen. Solvent was evaporated in the presence of vacuum using rotary evaporators. The dried powder was stored at  C overnight. Before injection, the reduced hydrocyanine powder was dissolved in 2 ml of deionized water making up the final concentration to 1 mg/ml [24].
C overnight. Before injection, the reduced hydrocyanine powder was dissolved in 2 ml of deionized water making up the final concentration to 1 mg/ml [24].2.2 Heat Inactivation of Staphylococcus Aureus
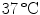 . Single colonies were picked with a sterile needle and used to inoculate a liquid LB culture that was incubated on a rotary shaker at 180 rpm at
. Single colonies were picked with a sterile needle and used to inoculate a liquid LB culture that was incubated on a rotary shaker at 180 rpm at 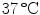 . When the density of the culture reached an OD
. When the density of the culture reached an OD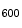 of 0.1, 1 ml of the bacterial culture was centrifuged at maximum speed in an Eppendorf centrifuge for 5 mins at room temperature. The supernatant was discarded and the pellet was suspended in 1 ml of phosphate buffered saline (PBS), pH 7.0. For inactivation, the bacteria were first heated to
of 0.1, 1 ml of the bacterial culture was centrifuged at maximum speed in an Eppendorf centrifuge for 5 mins at room temperature. The supernatant was discarded and the pellet was suspended in 1 ml of phosphate buffered saline (PBS), pH 7.0. For inactivation, the bacteria were first heated to 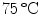 for 15 mins and then stored on ice.
for 15 mins and then stored on ice.2.3 Implant Preparation
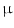 m) were used as biocompatible implants. Inflammatory porous glass implants were prepared by soaking the beads for 2 min in heat inactivated Staphylococcus aureus suspensions and left to dry under ambient conditions. Porous titanium discs of 7 mm diameter and 2 mm thickness were prepared from micro-beads by an injection molding and sintering procedure. Magnesium discs with a diameter of 5 mm and height of 2 mm were prepared by extrusion of a rod followed by cutting off individual discs. Poly-L-lactic acid beads with a diameter of 5 mm were purchased from Good Fellow, England.
m) were used as biocompatible implants. Inflammatory porous glass implants were prepared by soaking the beads for 2 min in heat inactivated Staphylococcus aureus suspensions and left to dry under ambient conditions. Porous titanium discs of 7 mm diameter and 2 mm thickness were prepared from micro-beads by an injection molding and sintering procedure. Magnesium discs with a diameter of 5 mm and height of 2 mm were prepared by extrusion of a rod followed by cutting off individual discs. Poly-L-lactic acid beads with a diameter of 5 mm were purchased from Good Fellow, England.2.4 Subcutaneous Implantations in Mice
2.5 In Vivo Imaging of the Oxidation Potential
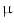 g of hydrocyanine solution was injected subcutaneously at the site of implantation. After 30 mins, fluorescent imaging was done in the near infrared spectrum using in vivo imaging system (IVIS200, Xenogen, USA). The excitation wavelength of hydrocyanines was 750 nm and the emission wavelength was 840 nm. Acquired images were corrected for the background using image math tool of living image software (version 4.3.1, Caliper life Sciences, 2012. In order to correct for the background, a region of interest was selected from a mouse without implants after addition of the fluorophore. The background value was calculated and automatically subtracted from the images by the software.
g of hydrocyanine solution was injected subcutaneously at the site of implantation. After 30 mins, fluorescent imaging was done in the near infrared spectrum using in vivo imaging system (IVIS200, Xenogen, USA). The excitation wavelength of hydrocyanines was 750 nm and the emission wavelength was 840 nm. Acquired images were corrected for the background using image math tool of living image software (version 4.3.1, Caliper life Sciences, 2012. In order to correct for the background, a region of interest was selected from a mouse without implants after addition of the fluorophore. The background value was calculated and automatically subtracted from the images by the software.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


