Chapter 22 Terpenes
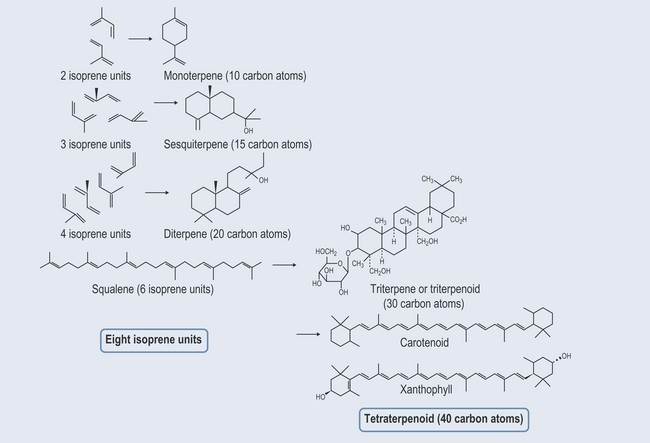
Terpenes are largely found as constituents of essential oils. They are mostly hydro-carbons. The building block is a five-carbon isoprene (CH2=C(CH3)—CH=CH2) unit (Figure 22.1). Terpene hydrocarbons have a molecular formula of (C5H8)n; the n dictates the number of units involved. Terpene hydrocarbons are classified according to the number of isoprene units:
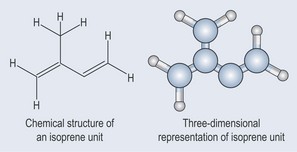
Figure 22.1 The chemical structure of the isoprene building block of terpenes and its three-dimensional representation.
• Lamiaceae (Labiatae): Melissa officinalis (lemon balm), Agastache rugosa (Huo Xiang), Rosmarinus officinalis (rosemary), Lavandula officinalis (lavender), Mentha spp. (mint, Bo He).
Monoterpenes
General properties of monoterpenes (Figure 22.2):
• Volatile oils: can therefore be administered as inhalations. Have very characteristic odours and are used in perfumes.
• Easily penetrate membranes as non-polar (and therefore lipophilic) compounds. This quality is utilized in aromatherapy.
• Generally, monoterpenes are antimicrobial (Gram positive and Gram negative; Trombetta et al 2005) and antifungal.
Iridoids
• Cardiovascular, stomachic, choleretic, antihepatotoxic activities and insect attractant and repellant properties.
• Examples of Actions
• Aucubin (Figure 22.4) is found in Eucommiae ulmoides (Du Zhong); harpagoside is found in Harpagophytum procumbens (devil’s claw). Both have anti-inflammatory properties.
• Valtrate is thought to be one of the compounds responsible for the sedative properties of Valeriana officinalis (valerian). It belongs to the class of iridoids associated with valerian and referred to as valepotriates.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree