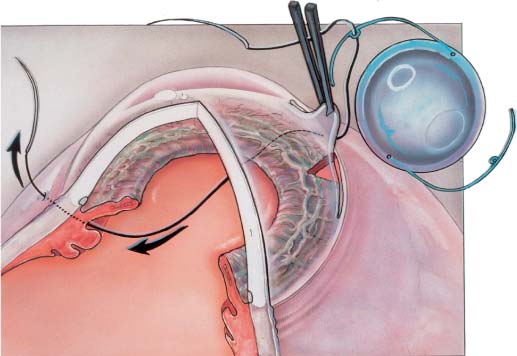
Chapter 22 Currently, most intraocular lenses (IOLs) are implanted at the time of cataract extraction. However, situations arise in which it may be beneficial for a patient to receive an IOL placed weeks, months, or even decades after the removal of a cataract. An IOL may be placed in an aphakic eye in a secondary IOL implantation procedure. As the average age of aphakic patients continues to rise, many patients find it increasingly difficult to tolerate aphakic spectacles or manipulate and care for their aphakic contact lenses. The overwhelming majority of these patients will benefit from secondary IOL implantation. A number of alternatives to aphakia now exist when faced with a planned or unplanned lack of capsular support during anterior segment surgery. The new IOL the surgeon can implant may be an iris fixated posterior chamber lens (PCL), a residual capsule-supported or transsclerally sutured (TS) PCL, or an anterior chamber lens (ACL). An unsuitable IOL may be removed from a pseudophakic eye at the same time a new one is placed in an IOL exchange procedure. Intraocular lens exchange is performed to replace a poorly tolerated IOL with another that will be better tolerated. Inflammation from an inappropriately sized or malpositioned ACL, PCL, or iris-supported IOL may cause chronic cystoid macular edema (CME). Glaucoma from peripheral anterior synechia (PAS) or uveitis-glaucoma-hyphema (UGH) syndrome secondary to malpositioned IOLs may also be occasionally seen. In addition, corneal decompensation from IOL-endothelial touch may be caused by either ACLs or iris supported PCLs. IOL exchange can also be performed for refractive reasons, to eliminate anisometropia due to inappropriate IOL power. Advantages of ACLs include ease of insertion and decreased intraoperative surgical time. Disadvantages include corneal endothelial cell loss, compromise of angle tissue, formation of peripheral anterior synechia, fibrosis of haptics into the angle, pupillary block, increased intraocular pressure, iris chafe, iritis, CME, and hyphema.1–10 Most of these complications were seen with closed-loop lenses. More recently designed flexible open-looped ACLs have decreased but not eliminated the above complications.5 Transscleral fixation of IOLs in the posterior chamber has a number of theoretical and practical advantages over ACLs. There are no lens elements in the angle, and transscleral fixation reduces the formation of PAS, fibrosis of haptics into the angle, or the development of the UGH syndrome. No lens elements traverse the pupil, decreasing the incidence of iris chafing, iritis, and CME. Finally, with the optic behind the iris there is a reduced chance of endothelial cell touch and pupillary block. However, both iris and TS lens techniques are technically more difficult to perform than ACL insertion. The increase in operative time can potentially increase the risk of intraoperative expulsive suprachoroidal hemorrhage, postoperative endophthalmitis, and prolonged recovery time. PCLs can be attached to the posterior iris surface by either the haptic or the optic.11–18 Potential disadvantages of iris sutured lenses include iris chafe, iritis, CME, pupillary block, pigment dispersion, glaucoma, and limited pupillary mobility. A small series of patients have documented some of these findings.17 Suture fixation of a lens to the posterior iris surface is also technically difficult through a limbal incision,19 in cases such as aniridia, or where there is limited iris tissue, or following trauma or multiple surgical procedures. TS PCLs have a number of advantages over iris-supported lenses. Using a transscleral fixation technique, the lens haptics come to rest in the ciliary sulcus by accurate placement of sutures through the scleral wall overlying the ciliary sulcus. With no suture or lens attachment to iris tissue, there is less iris chafe, pigment dispersion, iritis, and CME. In addition, pupillary motility remains unrestricted with a transscleral fixation technique. Transsclerally fixated IOLs are equally effective through open sky or limbal approaches, and can be particularly useful if inadvertent capsular rupture is encountered during routine cataract extraction. In addition, transscleral fixation of lenses is the preferred procedure in patients with aniridia, eyes with loss of iris tissue from trauma, multiple peripheral iridectomies or large sector iridectomies, and in patients having extensive PAS formation, shallow anterior chambers, or glaucoma. In designing a lens and surgical technique for implantation and transscleral fixation, a number of anatomic factors must be considered. The diameter of the ciliary sulcus and its relation to external landmarks are critical anatomic considerations. The ciliary sulcus measures 11.17 mm horizontally and 10.83 mm vertically, and corresponding corneal diameters are 11.67 mm and 10.97 mm.20 Needles passed through the ciliary sulcus and perpendicular to the scleral surface exit 0.46 mm horizontally and 0.83 mm vertically from the posterior limbus.21 As clinical experience has shown, complications of TS PCL fixation are due to inappropriate IOL design and insertion technique. Based on a better understanding of ocular anatomic features, IOL and technique modifications have been recommended to address the observed complications and to improve the safety and effectiveness of TS PCLs. It is critical that careful preoperative evaluation and surgical planning be performed prior to initiation of transscleral suture fixation techniques.22 Primary TS PC IOL implantation candidates are those patients undergoing routine cataract surgery whose surgery is complicated by unexpected posterior capsular rupture, vitreous loss, and vitrectomy. At the conclusion of vitrectomy these patients are found to have inadequate residual anterior capsular support for sulcus fixation of a standard PC IOL. These cases are discussed below. Suitable candidates for secondary IOL implants are aphakic patients who are either spectacle or contact lens intolerant. Pseudophakic patients with chronic CME, secondary glaucoma due to PAS, or corneal endothelial embarrassment with endothelial cell loss are also good candidates for secondary IOL with removal of the offending IOL. All patients should undergo a complete ocular examination prior to surgery. Retinal abnormalities should be ruled out with careful ophthalmoscopy. If there is clinical suspicion of a macular abnormality, fluorescein angiography should be performed. IOL calculations computed with accepted formulas using keratometry readings and axial length as measured by A-scan ultrasonography need to be performed even if the aphakic contact lens or spectacles refraction is known. Gonioscopy and endothelial cell counts are crucial to surgical planning. Careful surgical planning cannot be overemphasized.23 Three areas require careful consideration: astigmatism, endothelial cell assessment, and position and type of IOL implant. Preoperative keratometry should act as a guide to the incision location in the presence of significant astigmatism. In general, the incision should be placed in the steeper meridian of the preoperative cylinder. For example, if two diopters of “with-the-rule” astigmatism exist preoperatively, the surgeon should place the incision over the 90-degree meridian. Postoperative wound slip is a well-known phenomenon occurring 6 weeks to 4 months following surgery when the incision is larger than 7 mm in cord length. By centering the incision over the steep meridian, wound slip with a compensatory decrease in cylinder will occur in that meridian. Occasionally this means that the incision is centered over the temporal area, necessitating a temporal approach or an approach at an oblique axis. The improvement in overall corneal sphericity, however, outweighs the inconvenience of operating in a meridian that is not centered superiorly. Endothelial cell counts of less than 700 cells per mm3 may preclude secondary IOL implantation. Regional endothelial cell counts may be useful in minimizing surgical trauma. The area with the lowest cell count should be avoided in making the incision, especially if central endothelial cell counts are borderline. In cases of decompensating corneas with low cell counts, consideration should be given to performing penetrating keratoplasty with secondary IOL implantation or exchange. Gonioscopy should be performed in each patient to determine the location of synechiae, iridotomies, IOL haptics, and vitreous. The presence of PAS may greatly influence the ultimate type and position of the IOL used. The haptics of ACLs should be placed in the angle in a location clear of synechia. Additionally, any iridectomy sites should be avoided. The presence of a patent peripheral iridectomy should be ascertained preoperatively. When patency is in doubt, an additional iridectomy should be performed. The presence and location of vitreous in the anterior chamber must be noted, as this will affect the extent of the vitrectomy to be performed during the procedure. The pupil is then dilated and the amount of peripheral capsule support determined. The choice of style of IOL implant is largely a question of the surgeon’s personal preference and the degree of capsular support. IOLs should (1) be stable in position over the long term, (2) minimize contact with the anterior chamber angle, and (3) have sufficient iris-to-implant clearance so that iris chafing will not occur. If anterior chamber IOLs are to be used, flexible open-loop IOLs with four-point fixation haptics are preferred. The sizing of these lenses is critical to minimize corneal endothelial cell trauma as well as angle complications. When adequate capsular support is present, a single-piece allpolymethylmethacrylate (PMMA) lens or multipiece PCL can be inserted into the ciliary sulcus utilizing existing peripheral or central capsular support. When the entire capsule is intact, viscoelastic materials and blunt dissection can occasionally be used to “open” the capsular bag, and following polishing of the posterior capsule the lens can be inserted into the lens capsule. If no capsular support is present and a PCL is preferred, a transscleral fixation technique can be employed or a PCL fixated to the iris. Sizing again becomes a critical issue when placing the PCL into the ciliary sulcus. One-piece lenses greater than 12.5 mm in overall diameter are too large to fit into the ciliary sulcus and will often override the sulcus onto the pars plicata with the haptics resting upon the ciliary processes and even out onto the ora serrata. One-piece all-PMMA PC IOLs with eyelets on their haptics are particularly well suited for transscleral fixation, and techniques for their use are discussed below. Careful presurgical planning is crucial to the success of any secondary IOL procedure. In this way endothelial trauma may be minimized, excessive astigmatism avoided, and stable fixation of the IOL achieved. Many techniques have been described for transscleral fixation of PCLs.22,23 The following technique is the one the authors find both easiest and most consistent. The pupil is dilated with cyclopentolate hydrochloride 1.0% and phenylephrine hydrochloride 2.5%. A Flieringa ring may be used to lend support to the globe, especially if a vitrectomy is anticipated. Two conjunctival peritomies are made 180 degrees apart in the axis of the steep meridian with Wescott scissors. Wetfield cautery is used to attain hemostasis of the surgical beds. A 7- to 8-mm scleral, grooved, two-plane incision is made in the meridian of the positive corneal cylinder. A paracentesis is made with a 22-degree knife three clock hours away from the initial scleral incision, and the anterior chamber is entered through the scleral incision with a keratome. An automated core vitrectomy is performed if necessary, and the eye is filled with viscoelastic. Alternatively, a pars plana vitrectomy can be performed using an anterior chamber maintainer through the limbal paracentesis. A one-piece PMMA PCL is selected with an optic size of 6.5 mm or greater, an overall length of 12.0 to 12.5 mm, a biconvex optic, and positioning eyelets on the haptics to assure stable suture fixation and symmetrical lens placement (e.g., Alcon CZ70BD, Storz P66UV). One of the long needles of a double-armed 10-0 polypropylene suture (e.g., Ethicon CTC-6L, Ethicon STC-6, or Alcon PC-7) is passed through the eyelet on the inferior haptic of the PCL (Fig. 22–1). The superior wound is opened to its full extent, and the needle is then passed through this wound, over the superior iris, through the pupil, under the inferior iris, through the ciliary sulcus, and out through the sclera, exiting 0.75 mm from the limbus where the inferior conjunctiva had previously been recessed. The other needle is passed in a similar fashion 1.5 to 2.0 mm adjacent to the previous needle’s exit site. FIGURE 22–1 In placing a transsclerally sutured posterior chamber lens (TS PCL), one of the long needles of a double-armed 10-0 polypropylene suture is passed through the eyelet on the distal haptic of the PCL. The needle is then passed through the wound, over the proximal iris, inside the eye through the pupil, under the distal iris, through the ciliary sulcus, and out through the sclera, exiting 0.75 mm from the limbus. The second needle is passed in similar manner.
TECHNIQUES OF PRIMARY AND
SECONDARY TRANSSCLERAL
FIXATION OF POSTERIOR
CHAMBER INTRAOCULAR LENSES
ANTERIOR CHAMBER INTRAOCULAR LENSES
TRANSSCLERAL FIXATION OF POSTERIOR CHAMBER INTRAOCULAR LENSES
ANATOMIC CONSIDERATIONS
PATIENT SELECTION AND PREOPERATIVE EVALUATION
SURGICAL PLANNING AND CHOICE OF INTRAOCULAR LENS
SURGICAL TECHNIQUE OF SECONDARY TRANSSCLERAL FIXATED INTRAOCULAR LENSES
PREPARING THE DISTAL HAPTIC
PREPARING THE PROXIMAL HAPTIC
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree