1. Describe the equipment used in aseptic manipulations and how to remove an air bubble from the syringe barrel. 2. Describe how to set up equipment in a laminar airflow workbench and the proper use of sterile 70% isopropyl alcohol and swabs. 3. Demonstrate the following procedures: a. “Staging” or preparing the admixture for the pharmacist to check b. Transferring medication using a vial with powder c. Reconstituting medication from a vial using proper diluent d. Transferring medications from ampules using a syringe and filter needle e. Adding medication to a plastic bag Before any manipulations can occur, certain tasks must be performed. Handwashing and garbing using the USP 797 guidelines are essential as well as cleaning the LAFW (see Chapter 6 for procedures). Technicians must understand these procedures and follow them in order to prevent contamination and subsequently avoid harm to the patient. We will discuss various techniques used in aseptic preparation, as well as manipulation of the equipment, in this chapter. Ampule necks and vial tops should be disinfected with sterile 70% IPA swabs. This should be done by making one gentle stroke across the surface, disposing of the swab, and allowing the area to dry. These surfaces are considered critical sites because they are a fluid pathway surface. They should be wet for at least 10 seconds and allowed to dry so that microorganisms are eliminated (discussed in Chapter 6). Equipment, such as needles, syringes, and tubing, is packaged in protective covering from the manufacturer and is disposable. When inserting a needle into the rubber closure on a vial, the needle must never be “stabbed” into it. This can cause coring, which is when small pieces of the rubber closure get pushed into the solution and then possibly added to the bag. The needle should be placed at an angle with the bevel up. This will force the pieces away from the bevel. See Figure 7-1 for the step-by-step process to prepare a syringe. • Perform all calculations or research required to prepare the medication ordered. • Perform proper hand hygiene, garbing, and gowning procedure. • Spray hands with 70% IPA or disinfectant, allow to dry, and don sterile gloves. • Spray gloved hands with alcohol, and allow to dry. • Perform cleaning procedure for the LAFW. • All supplies used in the DCA should be gathered and decontaminated by spraying or wiping the outer surface with sterile 70% IPA or removing the outer packaging at the edge of the DCA as it is entered into the aseptic workspace. This will aid in removing dust particles and any other contaminates. • Attach the syringe to the needle inside the DCA without contact contamination or interruption of first air. • Disinfect all critical sites using sterile 70% IPA alcohol wipes, and wait at least 10 seconds before use. • Withdraw the correct volume of medication from the vial by injecting an equal or less amount of air into it first. (For example, if 2 mL are required, inject 2 mL of air into the vial and then withdraw the 2 mL of fluid.) Use the see-saw method to allow fluid and air to swap from the vial to the syringe. • Replace the protective needle cover, and remove any air bubbles by gently tapping on the syringe. Air bubbles will cause the reading on the syringe to be inaccurate because it allows the air to take up space, and it reflects in the final measurement.
Techniques for preparing intravenous admixtures
Introduction
Equipment used in aseptic manipulations
Removing an air bubble
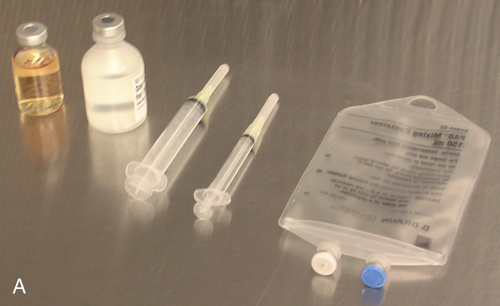
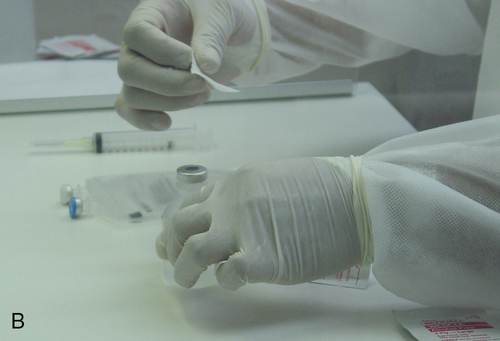
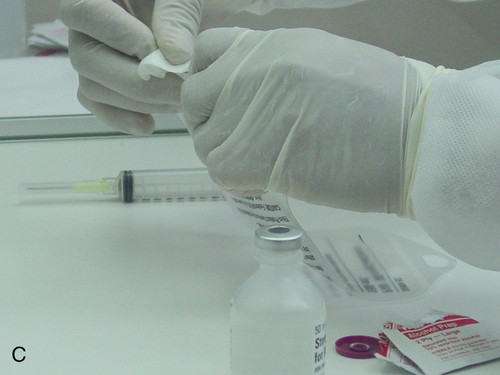
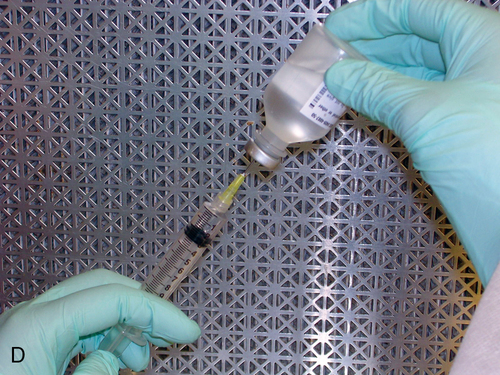
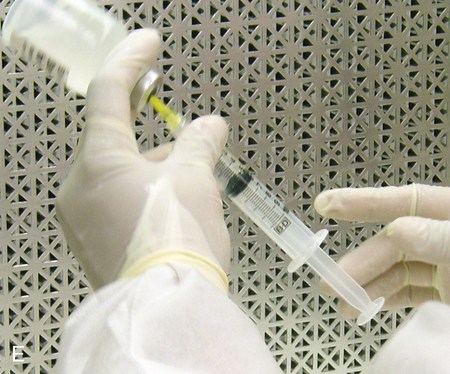
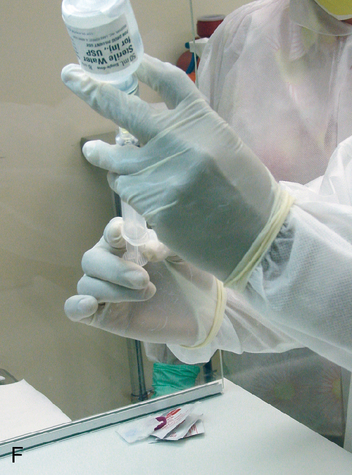
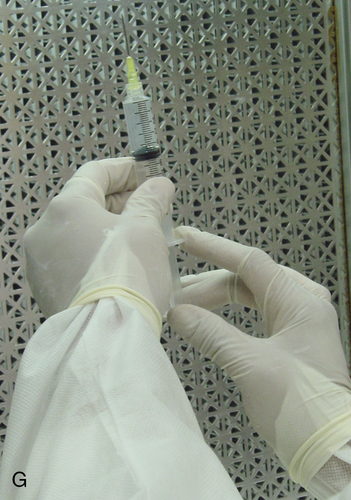
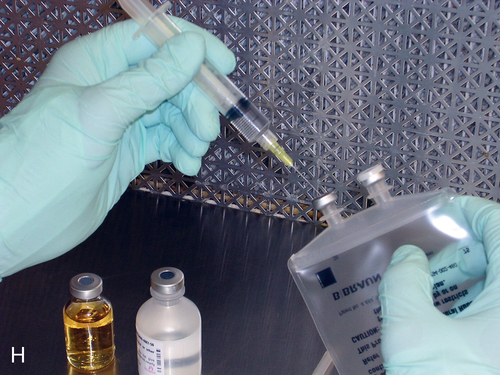
Setting up equipment in a laminar airflow workbench
Ante area
Buffer area
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Techniques for preparing intravenous admixtures
