CHAPTER 26 Systemic mastocytosis
Introduction
SM diagnosis is based on morphology and cannot be established on the basis of clinical findings alone. Therefore, the pathologist should be familiar with the diagnostic criteria defined for mastocytosis and also to recognize its mimickers.1,2 Important mimickers of mastocytosis are reactive states of mast cell hyperplasia and a few rare neoplastic hematological disorders such as tryptase-positive acute myeloid leukemia (AML) or myelomastocytic leukemia. Diagnostic criteria of SM are given in Box 26.1.
The major and two of four minor diagnostic criteria are morphology-based. Diagnosis of SM can be established if the major and one minor criterion are fulfilled. In cases lacking the major criterion, SM diagnosis can be established if at least three minor criteria are found.1,2 Most cases of SM can be easily diagnosed when focal compact infiltrates with a significant proportion of spindle-shaped mast cells are present. SM exhibiting exclusively round mast cells can be diagnosed after demonstration of an atypical immunophenotype with expression of CD25, which is not present in normal mast cells.3 If compact mast cell infiltrates are missing, diffusely scattered spindle-shaped mast cells with CD25 expression alone are not enough to establish a diagnosis of mastocytosis. In these patients, demonstration of the KITD816V mutation and/or chronically elevated serum tryptase enables diagnosis of mastocytosis since three of four minor criteria are fulfilled.4,5,6
BM is the main tissue where diagnosis of SM can be established. However, demonstration of compact mast cell infiltrates in extramedullary tissues like lymph node, spleen, liver and/or mucosa should also be regarded as strong indication for SM.7,8,9 Rarely, the diagnosis of mastocytosis is first established in the mucosa of the gastrointestinal (GI) tract and BM involvement is confirmed later. It is rather unlikely that pure GI form exists. In all patients with GI involvement, the meticulous investigation of the BM should be performed using immunohistochemistry and molecular biology. In a considerable proportion of SM patients, the degree of tissue infiltration is very low. The WHO 2008 classification of mastocytosis is given in Box 26.2.2 The approach to the diagnosis of mastocytosis is complex and considering its relatively low incidence, the diagnosis is usually more difficult than in most other hematological malignancies. The different forms of SM can only be recognized when the pathologist is aware of important clinical findings, especially the so-called ‘B-findings’, including organomegaly, and ‘C-findings’, indicating organ dysfunction due to widespread mast cell infiltration (Box 26.2).
Box 26.2 Classification of mastocytosis (WHO 2008)2
‘B’ findings
Molecular genetics aspects
Mast cells of most patients with SM carry the activating point mutation KITD816V of the c-kit gene.10 However, the frequency of KITD816V varies between subtypes of the disease. It has been found in almost 100% of patients with SM-AHNMD but only in about 50–60% of patients with aggressive SM and mast cell leukemia (MCL). Indolent SM assumes an intermediate position. Other than D816V point mutations of c-kit (e.g. D816Y or D816H) do occur but are rarely detected in SM.11 A considerable number of patients with SM-AHNMD were found to carry KITD816V not only in the SM but also in the AHNMD compartment of the disease. The frequency of KITD816V depends on the subtype of hematological disorder. SM-associated chronic myelomonocytic leukemia (CMML) exhibits KITD816V in almost all cases. The mutation is found both in the SM and the AHNMD compartment of the disease, which underlines a close clonal relationship between SM and CMML in the setting of SM-AHNMD. In SM associated with a myeloproliferative neoplasm with eosinophilia (MPNEo), KITD816V is usually not found in the MPNEo. Very surprisingly, it is also lacking in the SM compartment, although compact infiltrates of CD25+ mast cells are present in a few cases thus enabling the morphological diagnosis of SM.12 In other types of SM-AHNMD (SM with myelodysplastic syndrome (SM-MDS), SM-AML, and SM with myeloproliferative neoplasms (SM-MPN)), the incidence of KITD816V in the associated disorder varies between 20% and 60% of patients. Interestingly, it has been shown that in cases of SM-MPN (e.g. primary myelofibrosis), both mast cells and cells of neutrophilic lineage could carry both activating point mutations KITD816V and JAK-2V617F, further indicating the close relationship between SM and ‘AHNMD’.13
Important messages
Routine work-up of cases with suspected systemic mastocytosis
Bone marrow trephine biopsy
The adequate BMTB specimen should be above 2 cm in length. Antibodies against CD25, CD117 and tryptase should be applied.20,21,22,23 In cases of suspected SM-AHNMD further immunohistochemical stainings with appropriate antibodies depending on the subtype of the hematological disorder should also be performed. The tissue can also be used for demonstration of the KITD816V mutation, which is best detected if the biopsy is fixed in 5% buffered neutral formalin and mildly decalcified in ethylenediaminetetraacetic acid (EDTA) overnight. Peripheral blood (PB) and BM smears are crucial for differential diagnosis between aggressive SM (ASM) and aleukemic MCL or aleukemic from leukemic MCL, respectively.
General morphological aspects of SM
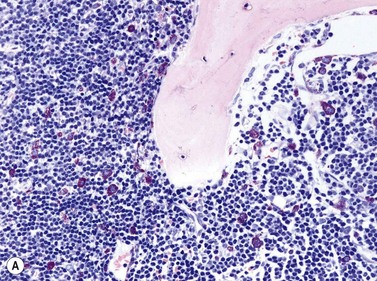
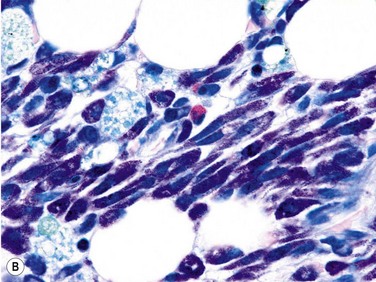
Size and cytological composition of compact (diagnostic) mast cell infiltrates varies greatly. Mast cells are not always dominant and may be obscured by follicle-like aggregates of lymphocytes. The lymphocyte-dominated infiltrates may mimic lymphocytic lymphoma, which is almost always accompanied by an increase in reactive (round, strongly metachromatic) mast cells posing considerable differential diagnostic problems in some cases (Fig. 26.1A).24 In contrast to mast cell hyperplasia, most SM cases exhibit at least one compact infiltrate consisting of slightly atypical often spindle-shaped and hypogranulated mast cells (Fig. 26.1B). Increased numbers of eosinophils are also usually found, admixed to lymphocytes and mast cells. Eosinophilic microabscesses are rarely detected. Focal increase in eosinophils within these compact infiltrates is only rarely accompanied by a significant eosinophilia in the hematopoietic islands. Stromal reaction shows almost always a dense network of reticulin fibers with the possibility to transform into collagen fibrosis. In some cases, the infiltrates may show striking variation in morphological appearance within the same biopsy specimen, ranging from large foci of collagen fibrosis with loosely scattered spindle-shaped mast cells to smaller lymphoid follicle-like structures with adjacent compact micronodules of round hypogranulated mast cells containing only slightly increased amounts of reticulin fibers. In larger infiltrates, there is also a significant angio-neogenesis with increase in small blood vessels. In most cases, mast cell infiltrates show a predominant peritrabecular localization leading to focal sclerosis of the adjacent bone. Cytomorphology of mast cells also varies greatly, ranging from cases with a predominance of spindle-shaped, markedly hypogranulated cells to cases containing exclusively round hypergranulated, mature-appearing mast cells, posing here the differential diagnosis of well-differentiated SM. The numbers of loosely scattered atypical mast cells are usually hard to estimate in Giemsa-stained sections (Fig. 26.2A), but they can be easily seen in CD25 immunostaining (Fig. 26.2B). It is crucial to apply all three antibodies: CD25, CD117 (KIT) and tryptase in all cases of suspected SM, in order to identify spindle-shaped, hypogranulated, fibroblast-like cells as mast cells, to recognize an aberrant immunophenotype with CD25-expression, and to be able to estimate the degree of the diffuse involvement of the BM aside from the compact mast cell infiltrates However, co-expression of tryptase and CD117 defines both normal/reactive and clonal-neoplastic mast cells of all stages of maturation and all degrees of atypia. Tryptase-expressing cells without CD117 are not mast cells but can be regarded as (neoplastic) basophils or myeloblasts. Such tryptase-positive CD117-negative cells are small to medium-sized and exclusively round. CD117-positive tryptase-negative cells are not mast cells but most likely BM progenitor cells of granulopoietic and/or erythropoietic lineage. Mast cells of high-grade subtypes like aggressive or leukemic SM may express some other aberrant markers like CD30 and/or CD33 that easily may lead to an erroneous diagnosis when the relevant mast cell-associated antibodies are not applied. In most patients with ISM or isolated SM of the bone marrow, the hematopoiesis is completely normal. Some signs of so-called inflammatory reaction like plasmacytosis, ceroid histiocytosis and eosinophilia are often present. In cases with aggressive SM or MCL, the hematopoiesis is often markedly reduced or almost completely effaced. It may be very difficult to assess or exclude the diagnosis of an ‘AHNMD’ (i.e., ASM-AHNMD or MCL-AHMD) in such cases. It is therefore strongly recommended to analyse BM and PB smears carefully in order not to miss an associated hematological malignancy. There are cases of ASM with subtotal involvement of the BM, where PB investigation revealed a diagnosis of CMML thus leading to the ultimate diagnosis of ASM-CMML. It is likely that ‘pure’ aggressive SM is a very uncommon disorder and ASM-AHNMD is the common setting (own unpublished observations).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree