Systemic Lupus Erythematosus
Shane M. Meehan, MBBCH
Key Facts
Terminology
Systemic autoimmune disease manifested by inflammation of skin, joints, kidneys and central nervous system
IgG autoantibodies to DNA, RNA, proteins, phospholipids
Etiology/Pathogenesis
Autoantibodies cause immune complex deposition, vasculitis, thrombotic microangiopathy
Multiple genetic risk factors
Microscopic Pathology
Glomerular pathology quite varied and is basis of ISN/RPS classification
Mesangial proliferation
Thickened GBM (extreme is wire loop)
Endocapillary hypercellularity
Necrosis, crescents
Thrombotic microangiopathy may dominate
IF: IgG deposits in glomeruli are essential for diagnosis
Usually accompanied by IgM, IgA, C1q, and C3 (full house)
TBM deposits in ˜ 50%
EM: Amorphous and structured deposits
Mesangial, subendothelial, and subepithelial deposits
Tubulointerstitial inflammation common
Top Differential Diagnoses
Cryoglobulinemia
MPGN, type I/III
Postinfectious GN
TERMINOLOGY
Abbreviations
Systemic lupus erythematosus (SLE); lupus nephritis (LN)
Synonyms
Lupus nephritis; lupus glomerulonephritis (GN)
Definitions
Systemic autoimmune disease manifested by inflammation of skin, joints, kidneys, and central nervous system, and with pathogenic IgG autoantibodies targeting nuclear, cell surface, and plasma protein self-antigens
ETIOLOGY/PATHOGENESIS
Autoimmune Disease
Autoantibodies to nuclear antigens in 95%: Double-stranded (ds) DNA (nucleosomes), Ro (a ribonucleoprotein), La (an RNA binding protein), Sm (a ribonucleoprotein)
Other autoantigens
C1q and membrane phospholipids such as phosphatidylserine
Laminin, heparan sulfate proteoglycans, and podocyte antigens
Some autoantibodies promote coagulation: Lupus anticoagulant, antiphospholipid antibodies, and ADAMTS13
Autoantibodies may be detectable years before disease onset
Breakdown of tolerance to self-antigens, trigger unknown
“Waste disposal” hypothesis
Defective phagocytosis of apoptotic cells leads to abnormal pathways of disposal of these cells
Abnormal disposal allows exposure of immune system to intracellular sequestered antigens
Exposure to these self-antigens activates self-reactive T and B cells and development of autoantibodies
Environmental Triggers and Aggravating Factors
Sunlight (UV)
Drugs: Hydralazine, procainamide, quinidine
Genetic Factors
10% have relatives with SLE
Higher concordance for monozygotic twins (25-69%) than dizygotic twins (1-2%)
Polymorphisms/mutations of > 25 genes increase risk in genome-wide association studies
C1, C2, or C4 deficiency, probably causing defective clearance of immune complexes and apoptotic cells
Fcγ receptor IIB (inhibitory receptor on B cells)
Toll-like receptors (TLR 7 and 9)
Interferon (IFN) and tumor necrosis factor (TNF) signaling
HLA-DR2 and 3
TREX1, a DNA-degrading enzyme
Pathogenesis
Immune complex (IC) formation and deposition in the kidney
Circulating IC trapped in capillaries and mesangium
Histone-rich nucleosomal antigens are trapped in glomeruli, possibly initiating immune complex (IC) deposition
In situ formation of IC due to planted or intrinsic antigen
Complement fixation
C3a and C5a attract neutrophils and macrophages
Endothelial activation by cytokines and complement
Inflammatory cells bind IC via Fc and complement receptors and are activated
Persistent or recurrent inflammation leads to changes in intrinsic glomerular cell numbers and function and matrix homeostasis
Thrombotic microangiopathy
Associated with lupus anticoagulant, antiphospholipid antibodies, and, rarely, antibodies to ADAMTS13
Arises independently of glomerular inflammation and IC
Vasculitic glomerulonephritis
Necrosis with little or no IC deposition
Endothelial damage by uncertain mechanism
Podocytopathy
Minimal change lesion, FSGS, or collapsing glomerulopathy
T-cell-mediated autoimmune reactivity may also play a role
Insights from Animal Models
Several spontaneous inbred mouse strains develop lupus-like glomerulonephritis, shown to be multigenic
NZB/NZW F1, MRL/lpr, BSXB
Many specific genetic deficiencies promote murine lupus in susceptible strains
C4, C3, FcγRIIB, CD19, fas, Ro
CLINICAL ISSUES
Epidemiology
Incidence
12-64/100,000 worldwide
˜ 80% have nephritis during the course of the disease
20% of patients have nephritis at onset
> 90% have nephritis at autopsy
Age
Peak incidence from 15-40 years
Also occurs in infants and the elderly
Gender
Female:male = 9:1
Ethnicity
Less frequent in Caucasians
1:250 females of African ancestry in USA
1:1,400 females of European ancestry in USA
Presentation
Gradual or sudden onset
Variable presentation, correlates with pathologic classification
Isolated hematuria: ISN/RPS class I, II, and III
Nephritic syndrome: Class III and IV
Isolated proteinuria: Class II and III
Nephrotic syndrome: Class IV and V
Acute renal failure: Class IV
Chronic renal failure: Class VI
Other features: Serositis, skin rash (malar, butterfly), anemia, arthritis, lymphadenopathy, thrombotic manifestations
Laboratory Tests
Autoantibodies
95% have autoantibodies to nuclear components
> 95% ANA (speckled pattern)
30-70% anti-dsDNA
30-50% anti-Ro (SSA)
30-40% anti-U1RNP
20-40% anti-Sm (highly specific for SLE)
40-50% anticardiolipin
15% antiphospholipid
30-50% anti-C1q
Associations with lupus nephritis strongest for antibodies to dsDNA and C1q
Complement levels
↓ C3 in 30-50%, ↓ C4 in 67-80%, more common in active disease
Treatment
Drugs
Immunosuppressive therapy used to decrease or arrest IC deposits, inflammation, and necrosis
Anti-B-cell antibodies targeting CD20 (rituximab) under evaluation
Transplantation
Clinically significant recurrence is rare (˜ 2%)
IF studies detect subclinical recurrence in ˜ 40%
Prognosis
Typically remitting and recurring course
25% develop end-stage renal failure
10-year renal survival is ˜ 75%
10-year patient survival is 72-98%
Common causes of death
Infection, especially pneumonia
Chronic renal failure
Neoplasms: B-cell lymphomas, lung carcinoma
General Approach
Kidneys are biopsied in SLE to determine type of renal disease, acute inflammatory activity, and extent of glomerular and tubulointerstitial scarring
Accurate assessment requires at least 20 glomeruli
2004 ISN/RPS classification is standard for lupus glomerulonephritis
MICROSCOPIC PATHOLOGY
Histologic Features
Lupus nephritis affects glomeruli, peritubular capillaries, muscular blood vessels, tubules, and interstitium with a spectrum of lesions
Glomeruli
Glomerular lesions determine ISN/RPS classification (classes I-VI)
Lesions can be active (acute) or chronic (sclerosing)
Focal (< 50% of glomeruli affected; III) or diffuse (≥ 50% of glomeruli affected; IV)
Segmental (part of a glomerulus) or global (> 50% of a glomerulus)
Distinction between III and IV is only by extent of these lesions (< or ≥ 50%, respectively)
Normal (I)
Requires mesangial IgG to diagnose lupus class I
Class I is seen in < 1% of biopsies for cause
Mesangial hypercellularity (II)
By itself is not considered an active lesion
Class II is seen in 10-15% of biopsies
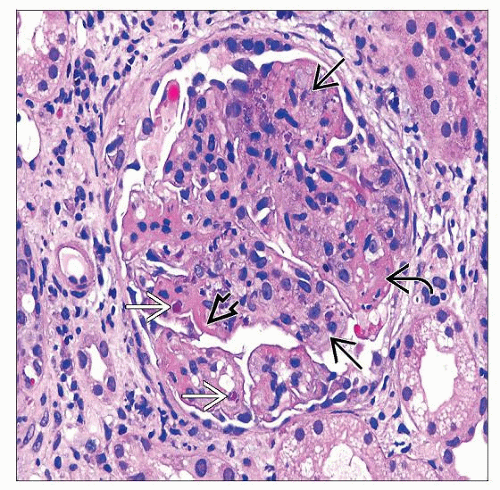
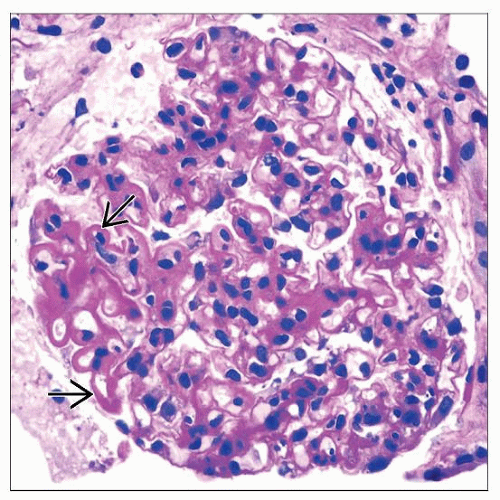
Endocapillary hypercellularity (III, IV)
Proliferation of intrinsic glomerular cells and accumulation of leukocytes with capillary luminal reduction
Class III is seen in 15-25% of biopsies
Class IV is seen in 40-60% of biopsies
Extracapillary hypercellularity (crescents) (III, IV)
Proliferation of parietal epithelium, accumulation of leukocytes, especially macrophages
Defined as > 2 cells thick, > 25% of circumference of capsule
Cellular or fibrous
GBM rupture associated with crescents
Karyorrhexis, fibrinoid necrosis (III, IV)
Prominent subendothelial deposits (“wire loops”) (III, IV)
Trichrome stain useful
Hyaline “pseudothrombi” (III, IV)
Hematoxylin bodies specific but rare (III, IV)
In vivo LE cell
Membranous lesions (V)
Diffuse thickening of GBM with granular subepithelial deposits (trichrome) with silver-positive “spikes”
Class V is seen in 10-15% of biopsies
Chronic glomerular lesions
Included in count of involved glomeruli for III vs. IV, if believed to be due to LN
Glomerular sclerosis, segmental or global
Glomerular fibrous adhesions
Fibrous crescents
GBM duplication
Variants
Some patients with minimal lupus (I-II) present with podocytopathy (minimal change disease and focal segmental glomerulosclerosis)
Focal segmental glomerular sclerosis can be a late scarring phase of lupus nephritis or secondary to loss of nephrons
Pathology may be dominated by lesions of thrombotic microangiopathy with little immune complex disease
Tubules and interstitium
Tubulointerstitial lesions are most commonly seen in association with class III and IV glomerular lesions
May be active or chronic
Occur ± IgG and C3 positive immune deposits
Active lesions are composed of T cells, macrophages, B cells, plasma cells, and neutrophils; with edema and tubulitis
Tubulointerstitial immune deposits may be associated with lymphoid aggregates
Chronic lesions are characterized by interstitial fibrosis and tubular atrophy
Occasional cases have predominately tubulointerstitial disease with only minimal glomerular disease (e.g., class II)
Vessels: 6 patterns observed
Normal (negative IF)
Uncomplicated vascular immune deposits
Normal-looking vessels with IgG, IgA, IgM, C3, and C1q by IF
Noninflammatory lupus vasculopathy
Hyaline deposits with Ig and C by IF
Necrotizing lupus vasculitis
Fibrinoid necrosis and leukocytoclasis ± immune deposits
Thrombotic microangiopathy
± class III or IV glomerular lesions
Arterial and arteriolar sclerosis
Nonlupus vasculopathy without immune deposits
ANCILLARY TESTS
Immunofluorescence
Detection of IgG deposits in glomeruli essential for diagnosis
Usually accompanied by IgA, IgM, C3, and C1q (“full house”)
Kappa and lambda equal
IgG1 and IgG3 are predominate subclasses
Fibrin in necrotizing lesions and thrombi
Glomerular patterns
Mesangial only (class I, II)
Capillary wall, focal or diffuse, coarse granular, elongated (class III, IV)
Capillary wall, diffuse, finely granular (class V)
Tubules
Granular IgG with variable other components common in tubular basement membrane (˜ 50% of cases)
Vessels
IgG and other components sometimes seen in small arteries, arterioles, and peritubular capillaries
Electron Microscopy
Amorphous electron-dense deposits present in glomeruli in varied locations and extent
Mesangium, subendothelium, subepithelium
Subepithelial deposits sometimes penetrate GBM
Substructure may be focally manifested as “thumbprint” pattern
Extraglomerular deposits of similar nature
Tubular basement membrane, Bowman capsule, peritubular capillaries, interstitium, small arteries
Glomerular endothelial cells have tubuloreticular structures
Clusters of vesicles and tubules of diameter 20-25 nm in endoplasmic reticulum
Consequence of elevated levels of interferon
DIFFERENTIAL DIAGNOSIS
Type II Mixed Cryoglobulinemia
Fibrillary substructure of deposits
Macrophage predominance in glomerular capillaries
Deposits can be sparse, usually little subepithelial
IgM-predominant immunoglobulin
“Pseudothrombi” do not distinguish
Often associated with hepatitis C virus
Membranoproliferative GN, Type I/III
C3-dominant deposits, IgG, and IgM in a band-like pattern along capillary walls
Usually no C1q
Lupus serologies negative
Postinfectious GN
IgG and C3 in a characteristic coarse granular (“lumpy-bumpy”) pattern, with hump-like deposits along subepithelium
Usually little C1q
Deposits in GBM (“humps”) do not have “spikes”
Idiopathic Membranous GN
Absence of subendothelial, mesangial, and extraglomerular deposits, and tubuloreticular inclusions
Antibodies to phospholipase A2 receptors
Deposits typically do not penetrate the GBM
Drug-induced LN
Many drugs implicated, e.g., propylthiouracil, isoniazid, hydralazine, procainamide, chlorpromazine
Few have anti-ds DNA antibodies or renal disease (5%)
High frequency of antihistone antibodies
Proof requires improvement after drug withdrawal
Lupus-like GN in HIV
Negative or low titer ANA and negative dsDNA antibodies by definition
50% diffuse, 45% focal, and 5% membranous pattern
˜ 20% of renal biopsies in HIV-infected patients
IgA Nephropathy
IgA-dominant deposits without tubuloreticular inclusions or extraglomerular immune deposits
C1q Nephropathy
Abundant mesangial immune deposits, with predominance of C1q
No clinical evidence of lupus
Pauci-immune Crescentic GN
Little or no endocapillary hypercellularity with little Ig and C deposition
Sjögren Syndrome
Distinction based on serology and clinical features
DIAGNOSTIC CHECKLIST
Clinically Relevant Pathologic Features
Histologic predictors of poor renal outcome
Class of LN broadly correlates with outcome but is less of a predictor because of tendency for class changes in follow-up biopsies
In general, mesangial and membranous lesions have better prognosis than focal or diffuse proliferative lesions
Amount of subendothelial deposits
Activity index
Chronicity index
ISN/RPS class, activity and chronicity indices, and presence of tubulointerstitial or vascular disease are essential for clinical management
Pathologic Interpretation Pearls
Lupus nephritis is in differential diagnosis of almost every renal biopsy
Heterogeneity of glomerular lesions by LM, glomerular and extraglomerular IgG, and endothelial tubuloreticular inclusions are most important diagnostic clues to LN
Hematoxylin bodies are pathognomonic but rare
GRADING
ISN/RPS Classification of Lupus Glomerulonephritis (2004)
Based on light and immunofluorescence microscopy; electron microscopy not required
≥ 20 glomeruli should be sampled for accurate classification
Tubulointerstitial disease and thrombotic microangiopathy scored separately
Classification requires distinction of active and chronic lesions and determination of extent of glomerular involvement by these injury processes
Single glomerulus may have both acute/active lesions and chronic/sclerosing lesions
Heterogeneity of interglomerular and intraglomerular changes makes classification of these lesions difficult
Transformation of glomerular lesions in repeat biopsies over months to years
Class II lesions upgrade to class III, IV, and V lesions in 33%
Class III lesions upgrade to class IV and V in 50%
Class IV lesions may upgrade to class V and VI lesions in 15%
Class III and IV lesions rarely downgrade to class II
Class V lesions may develop proliferative (class III or IV) lesions in ˜ 45% of cases
Majority of reported cases (78%) have no change of class in follow-up biopsies
Classes broadly correlate with outcome
Class I and II: Best survival
Class IV: Worse survival than III
Debate whether segmental and diffuse forms of class IV are different
Class V: Good long-term survival
Class VI: End-stage disease
Activity and Chronicity Indices
Histologic lesions scored 0, none; 1+, mild; 2+, moderate; 3+, severe and summed
Activity index (AI) (0-24)
Components: Endocapillary hypercellularity, neutrophils, fibrinoid necrosis/karyorrhexis, cellular crescents, “pseudothrombi”/“wire loops”
Scores for necrosis, karyorrhexis, and cellular crescents are doubled
Chronicity index (CI) (0-12)
Components: Glomerular sclerosis, fibrous crescents, interstitial fibrosis, and tubular atrophy
Reproducibility suboptimal
SELECTED REFERENCES
1. Morel L: Genetics of SLE: evidence from mouse models. Nat Rev Rheumatol. 6(6):348-57, 2010
2. Harley IT et al: Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 10(5):285-90, 2009
3. Seshan SV et al: Renal disease in systemic lupus erythematosus with emphasis on classification of lupus glomerulonephritis: advances and implications. Arch Pathol Lab Med. 133(2):233-48, 2009
4. Rahman A et al: Systemic lupus erythematosus. N Engl J Med. 358(9):929-39, 2008
5. Furness PN et al: Interobserver reproducibility and application of the ISN/RPS classification of lupus nephritis-a UK-wide study. Am J Surg Pathol. 30(8):1030-5, 2006
6. Hill GS et al: Class IV-S versus class IV-G lupus nephritis: clinical and morphologic differences suggesting different pathogenesis. Kidney Int. 68(5):2288-97, 2005
7. Weening JJ et al: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 15(2):241-50, 2004
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree