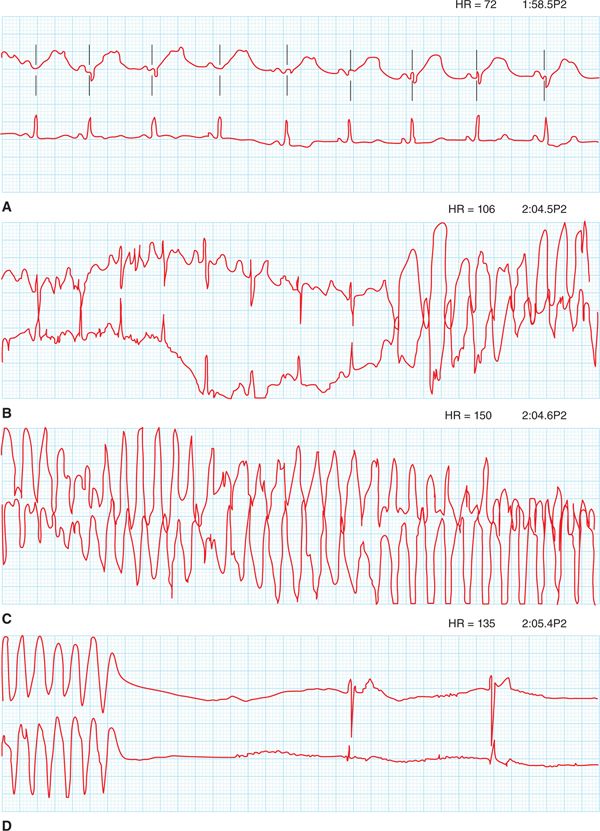
FIGURE 48-1 ECG showing sinus bradycardia at 48 beats per minute. There is a broad-based, notched T wave with a prolonged QT and T-wave inversions in anterior leads. Note that the T-wave notching can be seen in ≥3 leads.
FIGURE 48-2 This tracing starts in sinus rhythm with a prolonged QT. The T wave is broad-based and notched. Polymorphic VT is initiated after a premature ventricular contraction (PVC). This then self-terminates with a junctional escape rhythm.
CASE EXPLANATION
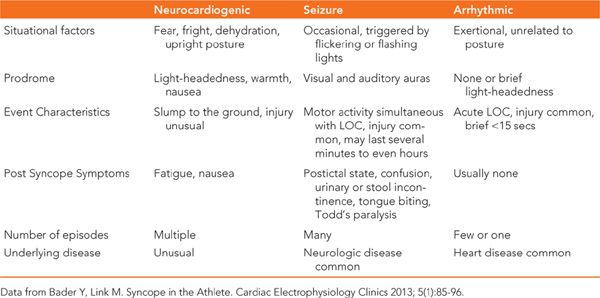
Although this patient was diagnosed as having a seizure disorder, it is important to note that these episodes of altered consciousness have been refractory to antiepileptic medications. Recently they occurred during exercise, raising concerns for alternative diagnoses. A seizure can of course be the result of a primary neurologic problem. However, seizure-like activity, or convulsive syncope, can be the result of cerebral hypoperfusion. This can result from common neurocardiogenic syncope or even fatal cardiac arrhythmias. In this case, the typical prodromal features of vasovagal syncope did not precede the loss of consciousness, making that an unlikely diagnosis. Syncope occurring during exercise is a strong predictor that the underlying problem is likely cardiac in origin. The scalp abrasion our patient suffered also supports the diagnosis of a cardiac arrhythmia which would result in a sudden drop in blood pressure and therefore the inability of a patient to protect their head as they fall. (Table 48-1 demonstrates features of cardiac and noncardiac syncope.1)
TABLE 48-1 Features of Cardiac and Noncardiac Syncope
• Exertional syncope is a precursor of sudden cardiac death (SCD). When identified, an appropriate workup should be initiated quickly to achieve a diagnosis and begin managing the patient.
• The differential diagnosis of cardiac syncope in this setting is seen in Table 48-2.
TABLE 48-2 Differential Diagnosis
Benign causes of syncope |
• Neurocardiogenic syncope |
• Postexertion collapse |
Life-threatening cardiac causes of syncope |
• Long QT syndrome (LQTS) |
• Catecholaminergic polymorphic ventricular tachycardia (CPVT) |
• Right ventricular outflow tract ventricular tachycardia (RVOT VT) |
• Brugada syndrome |
• Arrhythmogenic right ventricular dysplasia (ARVD) |
• Hypertrophic cardiomyopathy (HCM) |
• Coronary anomalies |
• Commotio cordis |
• Our patient’s ECG clearly demonstrates a prolonged QT with a broad based, notched T wave. In the absence of QT prolonging medications this is highly suggestive of an inherited long QT syndrome (LQT) type 1. Her Holter also confirmed that her syncope is secondary to torsades de pointes, which is defined as polymorphic ventricular tachycardia (VT) resulting from a prolonged QT.
EPIDEMIOLOGY
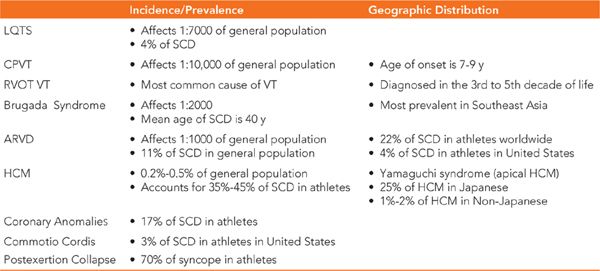
• Loss of consciousness in athletes, although rare, is devastating, and this is due to our growing understanding of SCD in athletes and its causes. Cardiac causes of syncope can be due to either structural heart disease or a primary arrhythmic condition and account for approximately 1% of all causes of syncope in the athlete. Details of incidence, prevalence, and geographic distribution are seen in Table 48-3.2–4
TABLE 48-3 Causes of Syncope in Athletes
• SCD is two and a half times more common in the competitive athlete than the general population, demonstrating its direct relationship to competitive sports.
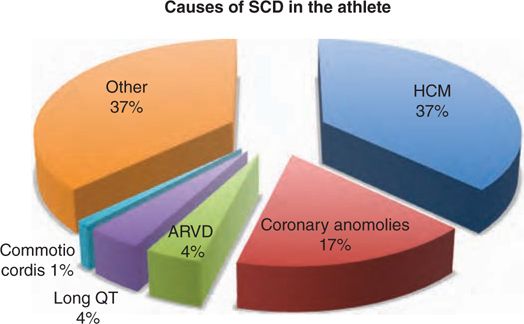
• The leading cause of SCD is hypertrophic cardiomyopathy (HCM) accounting for approximately 37% of cases followed by coronary anomalies and ion channelopathies, including long QT syndromes, which account for about 4% (Figure 48-3).
FIGURE 48-3 This pie chart displays causes of sudden cardiac death in athletes and their incidence.
ETIOLOGY AND PATHOPHYSIOLOGY
The majority of cardiac causes of syncope are either congenital or inherited (either autosomal recessive or autosomal dominant).
Long QT Syndrome
• This group of disorders is genetically inherited, affecting cardiac ion channels, which result in prolonged ventricular repolarization, which may lead to polymorphic ventricular tachycardia.
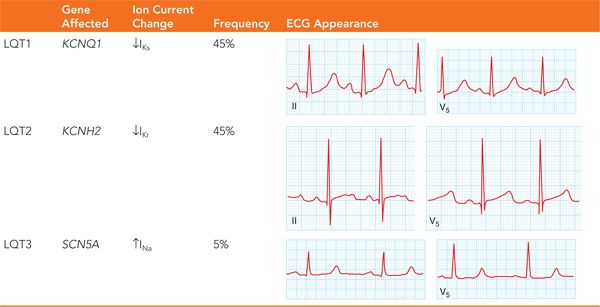
• There are 13 different types of inherited prolonged QT, all of which involve a different genetic mutation. The most common forms of long QT are types 1, 2, and 3 accounting for the majority cases.
 LQT1 results from a mutation in the α-subunit of the slow delayed rectifier potassium channel KCNQ1. These mutations result in reducing the amount of repolarizing current, thereby prolonging the action potential. This is the most common form of congenital long QT, making up one-third of cases. LQT1 is often triggered by exercise and has a specific association with swimming.
LQT1 results from a mutation in the α-subunit of the slow delayed rectifier potassium channel KCNQ1. These mutations result in reducing the amount of repolarizing current, thereby prolonging the action potential. This is the most common form of congenital long QT, making up one-third of cases. LQT1 is often triggered by exercise and has a specific association with swimming.
 LQT2 results from a mutation in the α-subunit of the rapid delayed rectifier potassium channel KCNH1. Similar to LQT1, the mutations of LQT2 result in prolongation of the action potential. LQT2 can be triggered by exercise but is more commonly triggered by an emotional event or an auditory stimulus.
LQT2 results from a mutation in the α-subunit of the rapid delayed rectifier potassium channel KCNH1. Similar to LQT1, the mutations of LQT2 result in prolongation of the action potential. LQT2 can be triggered by exercise but is more commonly triggered by an emotional event or an auditory stimulus.
 LQT3 is result of a mutation in the α-subunit of the sodium channel SCN5A. This leads to failure of the sodium channel to remain inactivated, which results in a prolonged action potential duration. LQT3 is less common than LQT1 and LQT2, but is more lethal.5 As in LQT1 and 2, LQT3 can also be triggered by exercise but more commonly occurs during sleep. Characteristic features of LQT1, LQT2, and LQT3 are seen in Table 48-4.
LQT3 is result of a mutation in the α-subunit of the sodium channel SCN5A. This leads to failure of the sodium channel to remain inactivated, which results in a prolonged action potential duration. LQT3 is less common than LQT1 and LQT2, but is more lethal.5 As in LQT1 and 2, LQT3 can also be triggered by exercise but more commonly occurs during sleep. Characteristic features of LQT1, LQT2, and LQT3 are seen in Table 48-4.
TABLE 48-4 The Long QT Syndromes
Catecholaminergic Polymorphic Ventricular Tachycardia
• Another genetic disease that may result in polymorphic VT in athletes is catecholaminergic polymorphic ventricular tachycardia (CPVT). It is characterized by cardiac electrical instability due to activation of the sympathetic nervous system. Unlike LQT syndrome, it is not related to prolongation of the QT interval.
 It is most commonly inherited in an autosomal dominant pattern resulting with mutations in the RYR2 gene, which encodes for the ryanodine receptor channel. This accounts for about 55% of CPVT.
It is most commonly inherited in an autosomal dominant pattern resulting with mutations in the RYR2 gene, which encodes for the ryanodine receptor channel. This accounts for about 55% of CPVT.
 Autosomal recessive inheritance is by mutations in the CASQ2 gene, which encodes calsequestrin, which is a calcium buffering protein of the sarcoplasmic reticulum and is responsible for about 2% of cases.6
Autosomal recessive inheritance is by mutations in the CASQ2 gene, which encodes calsequestrin, which is a calcium buffering protein of the sarcoplasmic reticulum and is responsible for about 2% of cases.6
• Mutations in the above receptors lead to calcium loading within myocardial cells and expose these patients to the risk of VT and ventricular fibrillation (VF) associated with acute emotion or exercise. Eighty percent of athletes with this disease have recurrent syncope, and thirty percent fall to sudden cardiac death.
Right Ventricular Outflow Tract Ventricular Tachycardia
• Right ventricular outflow tract ventricular tachycardia (RVOT VT) is the most common idiopathic VT in athletes. It most frequently occurs during emotional stress or exercise. Athletes usually present with palpitations and presyncope; however, it can infrequently present with syncope and exceptionally rarely present with sudden cardiac death.
• Similar to CPVT, the principal mechanism of RVOT VT is due to intracellular calcium overload. One of cyclic adenosine monophosphate’s (cAMP) roles is regulation of intracellular calcium. During exercise, cAMP levels rise, leading to an increase in intracellular calcium. This in turn, by a mechanism of triggered activity, leads to VT.
• There are two main phenotypic types of RVOT VT:
 Nonsustained repetitive monomorphic VT.
Nonsustained repetitive monomorphic VT.
 Paroxysmal exercise-induced sustained RVOT VT, which more commonly affects athletes.7
Paroxysmal exercise-induced sustained RVOT VT, which more commonly affects athletes.7
Brugada Syndrome
• Although Brugada syndrome may be acquired, it is most commonly inherited in an autosomal dominant fashion and is due to a loss of function mutation in the SCN5A gene.
• Athletes with Brugada syndrome usually present with unexplained syncope and, less frequently, as sudden cardiac death.
• VF in Brugada patients is usually triggered by hyperthermia and during times of higher vagal tone, often occurring during sleep.8 Because of their extensive training, athletes may have a higher baseline vagal tone, and during exercise rises in temperature may explain why athletes with Brugada are more likely to suffer from ventricular arrhythmias than nonathlete Brugada patients.
Arrhythmogenic Right Ventricular Dysplasia
• Arrhythmogenic right ventricular dysplasia (ARVD) is a genetic disorder with mutations in gene coding for desmosomal proteins, leading to fibrofatty replacement of myocardium. It is most often inherited in an autosomal dominant pattern.
• The right ventricle (RV) is affected in all but 5% of cases. There is left ventricular (LV) involvement in about 50% of cases and in a minority of cases only LV involvement.
• The “triangle of dysplasia” which is between the RV apex, the RV inflow tract, and the RV outflow tract is notoriously involved in ARVD.
• RVOT VT is the most common VT seen in these patients.3
Hypertrophic Cardiomyopathy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree