Surrogate Endpoints and Biomarkers
Science is facts. Just as houses are made of stones, so is science made of facts. But a pile of stones is not a house and a collection of facts is not necessarily science.
–Jules Henri Poincare (1854-1912).
Although clinical endpoints have been used to assess drugs for many centuries, there has been an increasing emphasis on finding surrogates for many of these endpoints in recent decades that can be used to evaluate a drug’s efficacy more rapidly and to bring new therapies to patients sooner. The evaluation of safety parameters in clinical trials includes measuring vital signs and conducting physical examinations, which again are clinical measures, but the development of laboratory measures over the past century and gradual introduction of newer assessments have expanded the evaluation of safety into an area where surrogates of clinical examinations are often used as adjuncts to assess the safety profile of drugs, in addition to using traditional clinical measures.
The primary focus of this chapter is to discuss issues relating to the expanding use of surrogate endpoints, although biomarkers and, to a much less degree, clinical endpoints are also discussed.
DEFINITIONS OF CLINICAL ENDPOINTS, BIOMARKERS, AND SURROGATE ENDPOINTS
Each of the following three definitions is quoted from an article (Biomarkers Definitions Working Group 2001) written by representatives of the Food and Drug Administration (FDA), National Institutes of Health (NIH), Pharmaceutical Research and Manufacturers of America, academia, and contract research organizations.
Clinical Endpoint
“Clinical endpoint: A characteristic or variable that reflects how a patient feels, functions or survives. Clinical endpoints are distinct measurements or analyses of disease characteristics observed in a study or a clinical trial that reflect the effect of a therapeutic intervention. Clinical endpoints are the most credible characteristics used in the assessment of the benefits and risks of a therapeutic intervention in randomized clinical trials.”
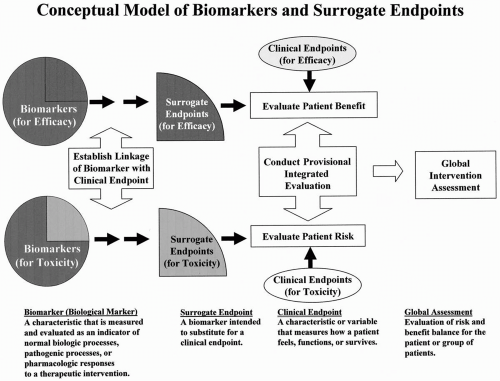 Figure 73.1 Conceptual model of biomarkers and surrogate endpoints. This figure was developed by the NIH Biomarker/Surrogate Endpoint Definitions Working Group. |
Biological Marker (Biomarker)
“Biological marker (biomarker): A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”
Surrogate Endpoint
“Surrogate endpoint: A biomarker that is intended to substitute for a clinical endpoint. A surrogate endpoint is expected to predict clinical benefit (or harm, or lack of benefit or harm) based on epidemiologic, therapeutic, pathophysiologic, or other scientific evidence.”
Surrogate endpoints are a subset of biomarkers. Although all surrogate endpoints can be considered biomarkers, it is likely that only a few biomarkers will achieve surrogate endpoint status. This is illustrated in the conceptual model of biomarkers and surrogate endpoints shown in Fig. 73.1. The relationship between biomarkers and surrogate endpoints is further detailed in Fig. 73.2.
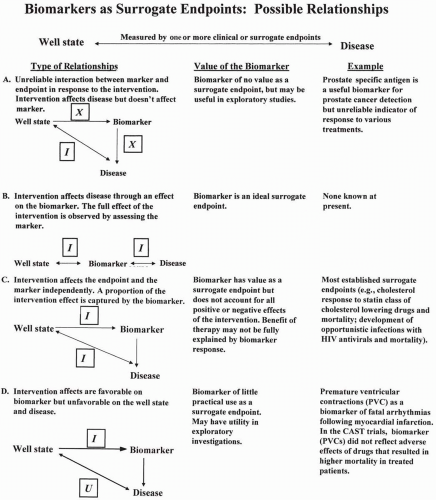 Figure 73.2 Four possible relationships of biomarkers as surrogate endpoints. HIV, human immunodeficiency virus; CAST, Cardiac Arrhythmia Suppression Trial; I, affected by intervention; X, not affected by intervention; U, unintended effect of intervention. This figure was developed by the NIH Biomarker/Surrogate Endpoint Definitions Working Group. |
CLASSIFYING BIOMARKERS AND SURROGATE ENDPOINTS
Because the plethora of biomarkers and surrogate endpoints can easily create confusion when simply spoken of and discussed as a group, it is useful to be able to differentiate among them by various factors. This is readily accomplished by classifying them according to several aspects, such as those reviewed in the following section.
Classification of Biomarkers and Surrogate Endpoints
Some of the main aspects that would need to be captured in creating a classification system would include the following:
Full name of the biomarker or surrogate endpoint
Therapeutic area (e.g., central nervous system)—some biomarkers and surrogates will fit multiple therapeutic areas
Disease and subtypes of disease when relevant (e.g., types of epilepsy)
Clinical endpoint that reflects how a patient feels, functions, or survives
Surrogate endpoint’s purpose (e.g., to assess safety or efficacy, or for other purposes)
Mechanism of action of the biomarker or surrogate endpoint (if known) and how the mechanism relates to the clinical endpoint
Degree of validity (see criteria in the “Validation of Surrogate Endpoints” section that can be used to determine this measure); validity could be graded on a four-point or other scale to indicate the overall degree of validity
If this information was published, then the appropriate references that would establish the above preclinical and clinical data used to establish validity, plus additional information (e.g., mechanism of action), would be included.
Another way of classifying biomarkers and surrogates would be based on where the therapy acts in the causal chain (see Fig. 73.2). These models have the advantage of illustrating whether a drug acts prior to or subsequent to the clinical effect.
Another option is to base a classification on how a treatment works in terms of its mechanism of action. A major difficulty with this approach is that the mechanism of most treatments is unknown. In theory, if one knew that a drug inhibited a specific enzyme that was responsible for its activity, and the enzyme was validated as a surrogate endpoint, then other drugs that acted on the same enzyme might not need to demonstrate an effect on the clinical endpoint. Although this purported surrogate would need to be validated, other drugs could eventually demonstrate similar enzyme activity and be approved based on having the same clinical effect. A newly studied statin drug, for example, that had similar enzyme activity against HMG-CoA Reductase as other statins might be approved for marketing based on that biochemical activity in clinical trials and not on having to demonstrate an effect on cholesterol levels. Note that cholesterol itself is a surrogate endpoint. This is highly theoretical and only presented to make the point that a mechanistic basis for classifying surrogates could be established, although the example given is unlikely to occur in practice.
TYPES OF SURROGATE ENDPOINTS
Biological, Chemical, and Procedure-based Surrogate Endpoints
While some clinical signs can be measured and used as surrogate endpoints (e.g., blood pressure), there are a wide variety of other types of surrogate endpoints that can be evaluated and used, such as those shown in Table 73.1.
Multiple Surrogates Often Exist for a Single Clinical Endpoint
In many situations, there is not just one surrogate for a clinical endpoint, but several. For example, a new drug to treat cancer may be tested for its effect on mortality, the true clinical endpoint. A surrogate could be the size of the tumor, and in that case, efficacy would be based on whether the drug affects the tumor size and, if so, by how much. Other surrogates could be time to progression of the tumor, time to onset of symptoms after treatment, or progression-free interval. Further surrogates could be a biochemical marker or a genetic marker that has been shown to denote efficacy of the drug. Even if the existence of such surrogates could not be used to obtain regulatory approval because of questions about the amount of evidence supporting its validation, the surrogate would provide a great benefit for the sponsor if the genetic, biochemical, immunological, or other data allowed one to choose easily the best compound or drug to advance (or even whether to advance the drug) in an early Phase 2 clinical trial.
Surrogates of Surrogates
While most discussions of surrogates deal with them as surrogates of the clinical endpoint, it is also possible that the surrogate is really a further surrogate of another, more direct surrogate endpoint. For example, the clinical endpoint for using tissue plasminogen activator (tPA) to treat restenosis is mortality at one
year post myocardial infarction (MI). However, the surrogate that was originally measured by Genentech and the resulting data submitted for marketing approval to the FDA-involved demonstration of the opening of coronary arteries as tPA dissolved the clots blocking the arteries (i.e., showing improved patency of coronary arteries after clot dissolution).
year post myocardial infarction (MI). However, the surrogate that was originally measured by Genentech and the resulting data submitted for marketing approval to the FDA-involved demonstration of the opening of coronary arteries as tPA dissolved the clots blocking the arteries (i.e., showing improved patency of coronary arteries after clot dissolution).
Table 73.1 Types of tests and other data that are able to be used as surrogate endpoints | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


