INTRODUCTION
The effects of malnutrition on the surgical patient are well characterized in the literature but are often overlooked in the clinical arena. Between 30% and 50% of hospitalized patients are malnourished. Protein-calorie malnutrition produces a reduction in lean muscle mass, alterations in respiratory mechanics, impaired immune function, and intestinal atrophy. These changes result in diminished wound healing, predisposition to infection, and increased postoperative morbidity. Although most healthy individuals can tolerate up to 7 days of starvation (with adequate glucose and fluid replacement), those subjected to major trauma, the physiologic stress of surgery, sepsis, or cancer-related cachexia require earlier nutritional intervention. Methods to identify those at greatest need for supplemental nutrition and to adequately address their needs are discussed in this chapter.
NUTRITIONAL ASSESSMENT
Nutrition screening is the process of identifying patients who are either malnourished or at risk for developing malnutrition. Major trauma and surgical stress alter the intake and absorption of nutrients, as well as their utilization and storage by the body. In select patients (eg, those with severe malnutrition as determined below), preoperative nutritional support has been shown to significantly reduce perioperative morbidity and mortality. Although most patients do not require this level of support, nutrition screening is imperative to identify the patient at high risk for malnutrition or its sequelae. A comprehensive nutritional assessment incorporates the initial history, physical examination, and laboratory testing to provide a snapshot of the patient’s recent nutritional health.
The history and physical examination are the foundation of nutritional assessment. A complete medical history is essential to identify factors that predispose the patient to alterations in nutritional status (Table 10–1). Chronic illnesses such as alcoholism are commonly associated with protein-calorie malnutrition as well as vitamin and mineral deficiencies. Previous operative procedures such as gastrectomy or ileal resection may predispose to generalized malabsorption or isolated deficiency of iron, vitamin B12, or folate. In most cases, the possibility of malnutrition is suggested by the underlying disease or by a history of recent weight loss. Patients with renal failure who require hemodialysis lose amino acids, vitamins, trace elements, and carnitine in the dialysate. Cirrhotics often suffer from whole-body sodium overload despite being hyponatremic, and they are typically protein-deficient. Patients with inflammatory bowel disease, particularly those with ileal involvement, may develop protein deficiency due to a combination of poor intake, chronic diarrhea, and treatment with corticosteroids. Furthermore, alterations in the enterohepatic circulation of bile salts lead to fat, vitamin, calcium, magnesium, and trace element deficiencies. Approximately 30% of patients with cancer have protein, calorie, and vitamin deficiencies due either to the underlying disease or to antimetabolite chemotherapy (eg, methotrexate). Patients infected with HIV are frequently malnourished and have protein, trace metal (selenium and zinc), mineral, and vitamin deficiencies.
| History (Factors Predisposing to Malnutrition) |
|---|
| Absorption disorders (eg, celiac sprue) |
| AIDS |
| Alcoholism |
| Chronic renal insufficiency |
| Cirrhosis |
| Diabetes mellitus |
| Enteric obstruction |
| Inflammatory bowel disease |
| Malignancy |
| Past surgical history, especially involving gastrointestinal tract |
| Prolonged starvation |
| Psychiatric disorders (eg, anorexia nervosa) |
| Recent major surgery, trauma, or burn |
| Severe cardiopulmonary disease |
| Physical Examination |
| Skin: Quality, texture, rash, follicles, hyperkeratosis, nail deformities |
| Hair: Quality, texture, recent loss |
| Eyes: Keratoconjunctivitis, night blindness |
| Mouth: Cheilosis, glossitis, mucosal atrophy (eg, temporal wasting), dentition |
| Heart: Chamber enlargement, murmurs |
| Abdomen: Hepatomegaly, abdominal mass, ostomy, fistulas |
| Rectum: Stool color, perineal fistula, Guaiac test |
| Neurologic: Peripheral neuropathy, dorsolateral column deficit, mental status |
| Extremities: Muscle size and strength, pedal edema |
| Laboratory Tests |
| CBC: Hemoglobin, hematocrit, mean corpuscular volume (MCV), white blood cell count and differential, total lymphocyte count, platelet count |
| Electrolytes: Sodium, potassium, chloride, calcium, phosphate, magnesium |
| Liver function tests: AST (SGOT), ALT (SGPT), alkaline phosphatase, bilirubin, albumin, prealbumin, retinol-binding protein, prothrombin/INR |
| Miscellaneous: BUN, creatinine, triglycerides, cholesterol, free fatty acids, ketones, uric acid, calcium, copper, zinc, magnesium, transferrin |
A complete history of current medications is essential to alert caregivers to potential underlying deficiencies and drug-nutrient interactions. Although rarely the sole cause of malnutrition, certain over-the-counter herbal preparations can alter nutrient absorption. Agents containing ephedra and caffeine may be abused to induce excessive weight loss. Ginkgo and other preparations enhance cytochrome p450 metabolism of various drugs. Information about socioeconomic factors and a detailed dietary history may uncover other risk factors.
A careful physical examination begins with an overall assessment of the patient’s appearance. Patients with severe malnutrition may appear frankly emaciated, but more subtle signs of malnutrition include temporal muscle wasting, skin pallor, edema, and generalized loss of body fat. Protein status is evaluated from the bulk and strength of the extremity muscles and visible evidence of temporal and thenar muscle wasting. Cardiac flow murmurs may result from anemia. Vitamin deficiencies may be indicated by changes in skin texture, the presence of follicular plugging or a skin rash, corneal vascularization, cracks at the corners of the mouth (cheilosis), hyperemia of the oral mucosa (glossitis), cardiac enlargement, altered sensation in the hands and feet, absence of vibration and position sense (dorsal and lateral column deficits), or abnormal quality and texture of the hair. Trace metal deficiencies produce cutaneous and neurologic abnormalities similar to those associated with vitamin deficiency and may cause changes in the mental status of the patient.
Anthropometry is the science of assessing body size, weight, and proportions. Anthropometric measurements gauge body weight and composition with the intent of providing specific information about lean body mass and fat stores. Body composition studies may be used to determine total body water, fat, nitrogen, and potassium. Anthropometric measurements that can be easily performed in the clinic or at the bedside include determination of height and weight, with calculation of body mass index (BMI). Additional measurements such as arm span, body part summation, or knee-height measurement can also be used in nutrition assessment. More advanced techniques allow the clinician to assess the patient’s visceral and somatic protein mass and fat reserve. Accurate weight is important, as is current weight expressed as a percentage of ideal body weight. Ideal body weight values are taken from the 1983 Metropolitan Height-Weight Tables.
The BMI is used to measure protein-calorie malnutrition as well as overnutrition (eg, obesity). A BMI between 18.5 and 24.9 is considered normal in most Western civilizations. Overweight is defined as a BMI from 25 to 29.9, and a BMI greater than 30 defines obesity. BMI is calculated as follows:
Dual-energy x-ray absorptiometry (DEXA) is increasingly available in hospitals and can be used to assess various body compartments (mineral, fat, lean muscle mass). Most protein resides in skeletal muscle. Somatic (skeletal) protein reserve is estimated by measuring the mid-humeral circumference. This measurement is corrected to account for subcutaneous tissue, yielding the mid-humeral muscle circumference (MHMC). The result is compared with normal values for the patient’s age and gender to determine the extent of protein depletion. Fat reserve is commonly estimated from the thickness of the triceps skin fold (TSF). Reliability of anthropometric measurements is dependent on the skill of the person performing the measurement and is subject to error if performed by different caregivers on the same patient.
The visceral protein reserve is estimated from various serum protein levels, total lymphocyte count, and antigen skin testing (Table 10–2). The serum albumin level provides a rough estimate of the patient’s nutritional status but is a better prognostic indicator than tool for nutritional assessment. Serum albumin less than 3.5 mg/dL correlates with increased perioperative morbidity and mortality and increased length of hospital stay. Because albumin has a relatively long half-life (20 days), other serum proteins with shorter half-lives have greater utility for assessing response to nutritional repletion. Transferrin has a shorter half-life of 8-10 days and is a more sensitive indicator of adequate nutrition repletion than albumin. Prealbumin has a half-life of 2-3 days, and retinol-binding protein has a half-life of 12 hours. Unfortunately, their serum levels are also influenced by other factors, limiting their utility in assessing nutritional status or repletion.
| Clinical & Laboratory Parameters | Extent of Malnutrition | |||
|---|---|---|---|---|
| Mild | Moderate1 | Severe1 | ||
| Albumin (g/dL) | 2.8-3.5 | 2.1-2.7 | < 2.1 | |
| Transferrin (mg/dL) | 200-250 | 100-200 | < 100 | |
| Prealbumin (mg/dL) | 10-17 | 5-10 | < 5 | |
| Retinol-binding protein (mg/dL) | 4.1-6.1 (normal) | < 4.1 | ||
| Total lymphocyte count (cells/μL) | 1200-2000 | 800-1200 | < 800 | |
| Creatinine-height index (%) | 60-80 | 40-60 | < 40 | |
| Ideal body weight (%) | 80-90 | 70-80 | < 70 | |
| Weight loss/time | < 5%/month | < 2%/week | > 2%/week | |
| < 7.5%/3 months | > 7.5%/3 months | |||
| < 10%/6 months | > 10%/6 months | |||
| Skin antigen testing (No. reactive/No. placed) | 4/4 (normal) | 1-2/4 (weak) | 0/4 (anergic) | |
| Anthropometric Measurements | Male | Female | ||
| Triceps skin fold (mm) | ≤ 12.5 | ≤ 16.5 | ||
| Mid-humeral circumference (cm) | > 29 | > 28.5 | ||
Immune function may be assessed by hypersensitivity skin testing as well as total lymphocytic count, a reflection of T- and B-cell status. Subcutaneous injection of common antigens provides a semiobjective assessment of the antibody-mediated immune response, commonly impaired in malnourished patients. A low total lymphocyte count (TLC) correlates directly with the degree of malnutrition, though the count may be altered by infection, chemotherapy, and other factors, thus limiting its usefulness.
Indices provide a means of risk-stratification and objective comparison among patients (Table 10–3). Additionally, many nutritional indices have been prospectively validated and can provide prognostic information to further guide nutrition support services. Along with the BMI, these indices can assist surgeons in determining the correct timing for intervention and the progress being made toward the goal of adequate nourishment.
| Body Mass Index (BMI) | |
|---|---|
| BMI = weight (kg)/[height (m)]2 = 703 × weight (lbs)/[height (in)]2 | |
| Normal | 18.5-24.9 |
| Overweight | 25-29.9 |
| Obese | 30-40 |
| Morbid obesity | > 40 |
| Prognostic Nutritional Index (PNI) | |
| PNI = 158 – [16.6 × Alb1] − [0.78 × TSF2] − [0.2 × TFN3] − [5.8 × DH4] | |
| Note: for DH, > 5 mm induration = 2; | |
| 1-5 mm induration = 1; | |
| anergy = 0 | |
| Risk for complications: | |
| Low | < 40% |
| Intermediate | 40-49% |
| High | > 50% |
| Nutrition Risk Index (NRI) | |
| NRI = [15.19 × Alb] + 41.7 × [actual weight (kg)/ideal weight (kg)] | |
| Well-nourished | > 100 |
| Mild malnutrition | 97.5-100 |
| Moderate malnutrition | 83.5-97.5 |
| Severe malnutrition | < 83.5 |
| Malnutrition Universal Screening Tool (MUST) | |
| BMI score: weight loss score (unplanned weight loss): | |
| BMI > 20 (> 30 obese) = 0; weight loss < 5% = 0 | |
| BMI 18.5-20 = 1; weight loss 5.10% = 1 | |
| BMI < 18.5 = 2; weight loss >10% = 2 | |
| Acute disease effect: add 2 if there has been or is likely to be no nutritional intake for > 5 days. | |
| Risk of malnutrition: 0 = low risk; 1 = medium risk; ≥ 2 = high risk | |
| Geriatric Nutrition Risk Index (GNRI) | |
| GNRI = [1.489 × albumin (g/L)] + [41.7 × (weight/WLo)] | |
| The GNRI results from replacement of ideal weight in the NRI formula by usual weight as calculated from the Lorentz formula (WLo). Four grades of nutrition-related risk: major risk (GNRI < 82), moderate risk (GNRI 82-91), low risk (GNRI 92 to ≤ 98), no risk (GNRI > 98). | |
| Instant Nutritional Assessment Parameters (INA) | |
| Parameter: abnormal if | |
| Serum albumin < 3.5 g | |
| Total lymphocyte count < 1500/mm3 | |
Creatinine-height index (CHI) may be used to determine the degree of protein malnutrition, although it is less valid in patients who are severely catabolic or have chronic renal disease. A 24-hour urinary creatinine excretion is measured and compared with normal standards. CHI is calculated by the following equation:
The urinary excretion of 3-methylhistidine is a more precise measurement of lean body mass and associated protein stores. The amino acid histidine is irreversibly methylated in muscle. During protein turnover, 3-methylhistidine is not reutilized for synthesis, so the urinary excretion of this compound correlates well with muscle protein breakdown. Unfortunately, measurement of 3-methylhistidine is too expensive for use as a routine clinical test.
The prognostic nutrition index (PNI) has been validated in patients undergoing either major cancer or gastrointestinal surgery and found to accurately identify a subset of patients at increased risk for complications. Furthermore, preoperative nutritional repletion has been shown to reduce postoperative morbidity in this patient group. The PNI has been widely adapted to identify patients at risk in nonsurgical populations, who may benefit from nutritional support.
The nutrition risk index (NRI) was used by the VA TPN Cooperative Study Group for determining preoperative malnutrition, and it has since been prospectively cross-validated against other nutritional indices with good results. The index successfully stratifies perioperative morbidity and mortality using serum albumin and weight loss as predictors of malnutrition. Of note, the NRI is not a tool for tracking the adequacy of nutritional support, since supplemental nutrition often fails to improve serum albumin levels.
Subjective global assessment (SGA) is a clinical method that has been validated as reproducible and that encompasses the patient’s history and physical examination. It is based on five features of the medical history (weight loss in the past 6 months, dietary intake, gastrointestinal symptoms, functional status or energy level, and metabolic demands) along with four features of the physical examination (loss of subcutaneous fat, muscle wasting, edema, and ascites). Limitations of the SGA include its focus on chronic instead of acute nutritional changes and its enhanced specificity at the expense of sensitivity.
The mini-nutritional assessment (MNA) is a rapid and reliable tool for evaluating the nutritional status of the elderly. It is composed of 18 items and takes approximately 15 minutes to complete. The assessment includes an evaluation of a patient’s health, mobility, diet, anthropometrics, and a subject self-assessment. An MNA score of 24 or higher indicates no nutritional risk, while a score of 17-23 indicates a potential risk of malnutrition and a score of less than 17 indicates definitive malnutrition.
The malnutrition universal screening tool (MUST) detects protein-energy malnutrition and identifies individuals at risk of developing malnutrition using three independent criteria: current weight status, unintentional weight loss, and acute disease effect. The patient’s current body weight is determined by calculating the BMI (kg/m2). Weight loss (over the past 3-6 months) is determined by looking at the individual’s medical record. An acute disease factor is then included if the patient is currently affected by a pathophysiologic condition and there has been no nutritional intake for more than 5 days. A total score is calculated placing the patients in a low, medium, or high category for risk of malnutrition. A major advantage of this screening tool is its applicability to adults of all ages across all health care settings. Additionally, this method provides the user with management guidelines once an overall risk score has been determined. Studies have shown that MUST is quick and easy to use and has good concurrent validity with most other nutrition assessment tools tested.
The geriatric nutritional risk index (GNRI) is adapted from the NRI and is specifically designed to predict the risk of morbidity and mortality in hospitalized elderly patients. The GNRI is calculated using a formula incorporating both serum albumin and weight loss. After determining the GNRI score, patients are categorized into four grades of nutrition-related risk: major, moderate, low, and no risk. Finally, the GNRI scores are correlated with a severity score that takes into account nutritional status-related complications. The GNRI is not an index of malnutrition but rather a “nutrition-related” risk index.
The quickest and simplest measure of nutritional status is the instant nutritional assessment (INA). Serum albumin level and the TLC form the basis of this evaluation. Significant correlations between depressed levels of these parameters and morbidity and mortality have been noted. Not surprisingly, abnormalities of these same parameters are even more significant in critically ill patients. Although not designed to replace more extensive assessment measures, this technique allows for quick identification and early intervention in those individuals in greatest danger of developing complications of malnutrition.
Adult basal energy expenditure (BEE) is calculated using a modification of the Harris-Benedict equation (Table 10–4). This calculation includes four variables: height (cm), weight (kg), gender, and age (y). Total energy expenditure (TEE) represents the caloric demands of the body under certain physiologic stresses. TEE is determined by multiplying BEE by a disease-specific stress factor. TEE should be used to guide nutritional supplementation.
| Basal energy expenditure (BEE) in kcal/day | |
|---|---|
| Male: 66.4 + [13.7 × weight (kg)] + [5.0 × height (cm)] − [6.8 × age (yrs)] | |
| Female: 655 + [9.6 × weight (kg)] + [1.7 × height (cm)] − [4.7 × age (yrs)] | |
| Stress factors | |
| Starvation | 0.80-1.00 |
| Elective surgery | 1.00-1.10 |
| Peritonitis | 1.05-1.25 |
| Adult respiratory distress syndrome (ARDS) or sepsis | 1.30-1.35 |
| Bone marrow transplant | 1.20-1.30 |
| Cardiopulmonary disease (uncomplicated) | 0.80-1.00 |
| Cardiopulmonary disease with dialysis or sepsis | 1.20-1.30 |
| Cardiopulmonary disease with major surgery | 1.30-1.55 |
| Acute renal failure | 1.30 |
| Liver failure | 1.30-1.55 |
| Liver transplantation | 1.20-1.50 |
| Pancreatitis or major burns | 1.30-1.80 |
| Total energy expenditure (TEE) in kcal/day | |
| TEE = BEE × stress factor | |
Indirect calorimetry is the most accurate method for direct measurement of daily caloric requirements. Using a metabolic cart, oxygen consumption 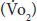 and carbon dioxide production
and carbon dioxide production 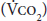 are directly measured from the patient’s pulmonary gas flow. Based on these measurements and the amount of nitrogen excreted in the urine, the resting energy expenditure (REE) can be derived using the Weir formula as follows:
are directly measured from the patient’s pulmonary gas flow. Based on these measurements and the amount of nitrogen excreted in the urine, the resting energy expenditure (REE) can be derived using the Weir formula as follows:
where 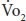 and
and 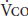 are expressed in milliliters per minute and urine nitrogen is in grams per minute. The utility of this technique is limited by the expense and cumbersomeness of the metabolic cart.
are expressed in milliliters per minute and urine nitrogen is in grams per minute. The utility of this technique is limited by the expense and cumbersomeness of the metabolic cart.
The respiratory quotient (RQ) is the ratio of carbon dioxide production to oxygen consumption in the metabolism of fuels by the body. When the RQ is 1, pure carbohydrate is being oxidized. Patients metabolizing lipids only will have an RQ of 0.67. Lipogenesis occurs in patients with excess caloric intake (overfeeding). When excessive calories are ingested or administered, the RQ is greater than 1 and can theoretically approach 9. The excess production of CO2 may impair ventilator weaning in patients, particularly those with intrinsic lung disease (eg, chronic obstructive pulmonary disease).
NUTRIENT REQUIREMENTS & SUBSTRATES
The body requires an energy source to remain in steady state. About 50% of the basal metabolic rate (BMR) reflects the work of ion pumping, 30% represents protein turnover, and the remainder is expended on recycling of amino acids, glucose, lactate, and pyruvate. Total energy expenditure is the sum of energy consumed by basal metabolic processes, physical activity, the specific dynamic action of protein, and extra requirements resulting from injury, sepsis, or burns. Energy consumed in physical activity constitutes 10%-50% of the total in normal subjects but decreases to 10%-20% for hospitalized patients. Energy expenditure and requirements vary, depending on the illness or trauma. The increase in energy expenditure above basal needs is about 10% for elective operations, 10%-30% for trauma, 50%-80% for sepsis, and 100%-200% for burns (depending on the extent of the wound). Metabolic energy can be derived from carbohydrates, proteins, or fats.
Carbohydrates are the body’s primary fuel source, accounting for 35% of total caloric intake. Each gram of enteric carbohydrate provides 4.0 kilocalories (kcal) of energy. Parenterally administered carbohydrates (eg, intravenous dextrose) yield 3.4 kcal/g.
Carbohydrate digestion is initiated by salivary amylase, and absorption occurs within the first 150 cm of the small intestine. Salivary and pancreatic amylases cleave starches into oligosaccharides. Surface oligosaccharidases then hydrolyze and transport these molecules across the gastrointestinal tract mucosa. Deficiencies in carbohydrate digestion and absorption are rare in surgical patients. Pancreatic amylase is abundant, and maldigestion of starch is unusual, even in patients with limited pancreatic exocrine function. Patients with diseases such as celiac sprue, Whipple disease, and hypogammaglobulinemia often have generalized intestinal mucosal flattening leading to oligosaccharidase deficiency and diminished carbohydrate uptake.
More than 75% of ingested carbohydrate is broken down and absorbed as glucose. Hyperglycemia stimulates insulin secretion from pancreatic β cells, which stimulates protein synthesis. Intake of 400 kcal of carbohydrate per day minimizes protein breakdown, particularly after adaptation to starvation. Cellular uptake of glucose, stimulated by insulin, inhibits lipolysis and promotes glycogen formation. Conversely, pancreatic glucagon is released in response to starvation or stress; it promotes proteolysis, glycogenolysis, lipolysis, and increased serum glucose. Glucose is vital for wound repair, but excessive carbohydrate intake or repletion with excessive amounts of glucose can cause hepatic steatosis and neutrophil dysfunction.
Proteins are composed of amino acids, and protein metabolism produces 4.0 kcal/g. Digestion of proteins yields single amino acids and dipeptides, which are actively absorbed by the gastrointestinal tract. Gastric pepsin initiates digestion. Pancreatic proteases, activated by enterokinase in the duodenum, are the principal effectors of protein degradation. Once digested, half of protein absorption occurs in the duodenum, and complete protein absorption is achieved by the mid-jejunum.
Protein absorption occurs efficiently throughout the small intestine; therefore, protein malabsorption is relatively infrequent even after extensive intestinal resection. Protein balance reflects the sum of protein synthesis and degradation. Because protein turnover is dynamic, the published requirements for protein, amino acids, and nitrogen are only approximations.
Total body protein in a 70-kg person is approximately 10 kg, predominantly in skeletal muscle. Daily protein turnover is 300 g, or roughly 3% of total body protein. The daily protein requirement in healthy adults is 0.8 g/kg body weight. In the United States, the typical daily intake averages twice this amount. Protein synthesis or breakdown can be determined by measuring the nitrogen balance (Table 10–5). Protein intake of 6.25 g is equivalent to 1 g of nitrogen. Nitrogen intake is the sum of nitrogen delivered from enteric and parenteral feeding. Nitrogen output is the sum of nitrogen excreted in the urine and feces, plus losses from drainage (eg, exudative wounds, fistula). Urea nitrogen losses are determined from a 24-hour urine collection. Fecal nitrogen loss can be approximated by 1 g/d, and an additional 2-3 g/d of nonurea nitrogen loss occurs in the urine (eg, ammonia). The accuracy of nitrogen balance calculations can be improved through measurement over several weeks. When losses of nitrogen are large (eg, diarrhea, protein-losing enteropathy, fistula, or burn exudate), measurements of nitrogen balance lose accuracy because of the difficulty in collecting secretions for nitrogen measurement. Despite these shortcomings, 24-hour urine collection is the best practical means of measuring net protein synthesis and breakdown.
The 20 amino acids are divided into essential amino acids (EAAs) and nonessential amino acids (NEAAs) depending on whether they can be synthesized de novo in the body. They are further divided into aromatic (AAAs), branched chain (BCAAs), and sulfur-containing amino acids. Only the l-isotype of an amino acid is utilized in human protein. Certain amino acids have unique metabolic functions, particularly during starvation or stress. Alanine and glutamine preserve carbon during starvation, and leucine stimulates protein synthesis and inhibits catabolism. Specific amino acids are addressed below.
As the respiratory fuel for enterocytes, glutamine plays an important role in the metabolically stressed patient. Following injury and other catabolic events, intracellular glutamine stores may decrease by over 50% and plasma levels by 25%. The decline of glutamine associated with injury or stress exceeds that of any other amino acid and persists during recovery after the concentrations of other amino acid have normalized. Supplementation with glutamine maintains intestinal cell integrity, villous height, and mucosal DNA activity and helps minimize reduction in numbers of T and B cells during stress.
Catabolic states are characterized by accelerated skeletal muscle proteolysis and translocation of amino acids from the periphery to the visceral organs. Glutamine accounts for a major portion of the amino acids released by muscle in these states. Intravenous supplementation with glutamine may improve neutrophil and macrophage function as well as decrease bacterial translocation across the intestinal mucosal barrier in burn and other critically ill patients. However, the utility of enteral supplementation remains controversial.
Arginine is a substrate for the urea cycle and nitric oxide production and a secretagogue for growth hormone, prolactin, and insulin. Arginine has been identified as the sole precursor of nitric oxide (endothelial-derived relaxing factor). The effects of arginine on T cells may be very important in maintaining the gut barrier. Formulas supplemented with arginine have been shown to improve nitrogen balance and wound healing, promote T-cell proliferation, enhance neutrophil phagocytosis, and reduce production of inflammatory mediators and infectious complications.
Lipids comprise 25%-45% of caloric intake in the typical diet. Each gram of lipid provides 9.0 kcal of energy. The introduction of fat to the duodenum results in secretion of cholecystokinin and secretin, leading to gallbladder contraction and pancreatic enzyme release. Reabsorption of bile salts in the terminal ileum (eg, the enterohepatic circulation) is necessary to maintain the bile salt pool. The liver is able to compensate for moderate intestinal bile salt losses by increased synthesis from cholesterol. Ileal resection may lead to depletion of the bile salt pool and subsequent fat malabsorption. Lipolysis is stimulated by steroids, catecholamines, and glucagon but is inhibited by insulin.
The body can synthesize fats from other dietary substrates, but two of the long-chain fatty acids (linoleic and linolenic) are essential. Insufficient intake of these essential fats leads to fatty acid deficiency and can be prevented by supplying a minimum of 3% of the total caloric intake as essential fatty acids.
The polyunsaturated fatty acids (PUFAs) are grouped into two families: ω-6 and ω-3 fatty acids. Linoleic acid is an example of the ω-6 PUFAs; ω-linolenic acid of the ω-3 PUFAs. Both linoleic and linolenic acid can be processed into arachidonic acid, a precursor in the synthesis of eicosanoids.
Eicosanoids are potent biochemical mediators of cell-to-cell communication and are involved in inflammation, infection, tissue injury, and immune system modulation. They also modulate numerous events involving cell-mediated and humoral immunity and can be synthesized in varying amounts by immune cells, particularly macrophages and monocytes.
Medium-chain fatty acids are not components of most oral diets but are widely used in enteral tube feedings. They are easily digested, absorbed, and oxidized and are not precursors to the inflammatory or immunosuppressive eicosanoids. Short-chain fatty acids, such as butyrate and to a lesser extent propionate, are utilized by colonocytes and provide up to 70% of their energy requirements. Since butyrate is not synthesized endogenously, the colonic mucosa relies on intraluminal bacterial fermentation to obtain this fuel.
In addition to the principal sources of metabolic energy (calories), many other substances are necessary to ensure adequate nutrition. Nucleotides are recognized as an important nutritional substrate in critically ill patients. Vitamins are essential for normal metabolism, wound healing, and immune function, and cannot be synthesized de novo. The normal requirements for vitamins are shown in Table 10–6. Vitamin requirements may increase acutely in illness. Trace elements are integral cofactors for many enzymatic reactions and are generally not stored by the body in excess of requirements.
| Enteral | Parenteral | |
|---|---|---|
| Electrolytes | ||
| Sodium | 90–150 meq | 90–150 meq |
| Potassium | 60–90 meq | 60–90 meq |
| Trace elements | ||
| Chromium1 | 5–200 μg | 10–15 μg |
| Copper1 | 2–3 mg | 0.3–0.5 mg |
| Manganese1 | 2.5–5 mg | 60–100 μg |
| Zinc | 15 mg | 2.5–5 mg |
| Iron | 10 mg | 2.5 mg |
| Iodine | 150 μg | … |
| Fluoride1 | 3 mg | … |
| Selenium1 | 50–200 μg | 20–60 μg |
| Molybdenum1 | 150–500 μg | 20–120 μg |
| Tin2 | … | … |
| Vanadium2 | … | … |
| Nickel2 | … | … |
| Arsenic2 | … | … |
| Silicon2 | … | … |
| Vitamins | ||
| Ascorbic acid (C) | 60 mg | 200 mg |
| Retinol (A) | 1000 μg | 3300 IU |
| Vitamin D | 5 μg | 200 IU |
| Thiamin (B1) | 1.4 mg | 6 mg |
| Riboflavin (B2) | 1.7 mg | 3.6 mg |
| Pyridoxine (B6) | 2.2 mg | 6 mg |
| Niacin | 19 mg | 40 mg |
| Pantothenic acid | 4–7 mg | 15 mg |
| Vitamin E | 10 mg | 10 IU |
| Biotin | 100–200 μg | 60 μg |
| Folic acid1 | 200 μg | 600 μg |
| Cyanocobalamin (B12) | 2 μg | 5.9 μg |
| Vitamin K3 | 70–149 mg | 150 μg |
| Minerals | ||
| Calcium | 1300 mg | 0.2–0.3 meq/kg |
| Phosphorus | 800 mg | 300–400 meq/kg |
| Magnesium | 350 mg | 0.34–0.45 meq/kg |
| Sulfur | 2–3 g | … |
Nucleic acids are precursors of DNA and RNA and are not normally considered essential for human growth and development. The need for dietary nucleotides increases in severe stress and critical illness. Nucleotides are formed from purines and pyrimidines, and their abundance is especially important for rapidly dividing cells such as enterocytes and immune cells. Immunosuppression has been reported in renal transplant patients being maintained on nucleotide-free diets. Dietary nucleotides are necessary for helper-inducer T-lymphocyte activity. Diets supplemented with RNA or the pyrimidine uracil have been shown to restore delayed hypersensitivity and augment both the lymphoproliferative response and IL-2 receptor expression. Nucleotides may facilitate recovery from infection. These substrates are often incorporated into enteral formulas as potential immunomodulators.
Vitamins A, D, E, and K are fat soluble and are absorbed in the proximal small bowel in association with bile salt micelles and fatty acids. After absorption, they are delivered to the tissues in chylomicrons and stored in the liver (vitamins A and K) or subcutaneous tissue and skin (vitamins D and E). Although rare, there are reports of toxicity from excessive intake of fat-soluble vitamins (eg, hypervitaminosis A from consuming polar bear liver). Fat-soluble vitamins participate in immune function and wound healing. For example, intake of vitamin A 25,000 IU daily counteracts steroid-induced inhibition of wound healing, largely through increases in TBG-β.
Vitamins B1, B2, B6, and B12, vitamin C, niacin, folate, biotin, and pantothenic acid are absorbed in the duodenum and proximal small bowel, transported in portal vein blood, and utilized in the liver and peripherally. Water-soluble vitamins serve as cofactors to facilitate reactions involved in the generation and transfer of energy and in amino acid and nucleic acid metabolism. Water-soluble vitamins have limited storage in the body. Because of their limited storage, water-soluble vitamin deficiencies are relatively common.
The daily requirements for the trace elements (Table 10–6) vary geographically depending on differences in soil composition. There are currently nine identified essential trace minerals (Fe, Zn, Cu, Se, Mn, I, Mb, Cr, Co). Trace elements have important functions in metabolism, immunology, and wound healing. Subclinical trace element deficiencies occur commonly in hospitalized patients and various disease states.
Iron serves as the core of the heme prosthetic group in hemoglobin and in the mitochondrial cytochrome respiratory process. Impaired cerebral, muscular, and immunologic function can occur in patients with iron deficiency before anemia becomes clinically evident. Particular attention should be paid to assessing iron stores in pregnant and lactating women.
Zinc deficiency is characterized by a perioral pustular rash, darkening of skin creases, neuritis, cutaneous anergy, hair loss, and alterations in taste and smell. Copper deficiency is manifested by microcytic anemia (unresponsive to iron), defective keratinization, or pancytopenia. Chromium deficiency presents as glucose intolerance during prolonged parenteral nutrition administration without evidence of sepsis. Selenium deficiency, which can occur in patients receiving parenteral nutrition for a prolonged period, is manifested by proximal neuromuscular weakness or cardiac failure with electrocardiographic changes. Manganese deficiency is associated with weight loss, altered hair pigmentation, nausea, and low plasma levels of phospholipids and triglycerides. Molybdenum deficiency results in elevated plasma methionine levels and depressed uric acid concentrations, producing a syndrome consisting of nausea, vomiting, tachycardia, and central nervous system disturbances.
Iodine is a key component of thyroid hormone. Deficiency is rare in the United States because of the use of iodinated salt. Chronically malnourished patients can become iodine-deficient. Since thyroxine participates in the neuroendocrine response to trauma and sepsis, iodine should be included in parenteral nutrition solutions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


