Surgical Management of Ovarian Carcinoma
Arlan F. Fuller Jr
Introduction: the Pathogenesis of Epithelial Ovarian Tumors
In the United States, ovarian carcinoma is the leading cause of genital tract cancer death among women and its incidence rises steeply with increasing age after menopause. Two of the major reasons for the high case fatality rate in ovarian carcinoma are the early spread of the disease and the absence of symptoms associated with early stage disease, leading to late diagnosis in the majority of patients. Two-thirds of patients have evidence of intra-abdominal spread to peritoneal surfaces or to the omentum at the time of diagnosis.
Much of this problem of silent transcoelomic dissemination of tumor arises from the pattern of histogenesis of epithelial ovarian cancer. Ninety-five percent of all ovarian carcinoma is epithelial in derivation, not arising from the germ cells (2.5%) or from the gonadal stroma (2.5%), but from the overlying serosal surface of the ovary. The pathogenesis is associated with ovulation, such that invagination of surface epithelium at the site of ovulation leads to the formation of an inclusion cyst thought to be the precursor of the typical forms of ovarian carcinoma. As the female pelvis is open to the outside environment through patent fallopian tubes, particulate carcinogens may be incorporated into the cysts. This behavior accounts for the increased risk of ovarian carcinoma known to exist in women who dust their perineum with talc and for the decreased risk associated with prior tubal ligation or inhibition of ovulation with the use of oral contraceptives.
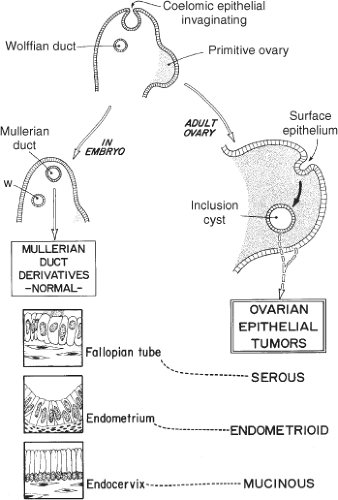
Formation of the epithelial inclusion cyst deep to the ovarian cortex is associated with appearance of immunohistochemical markers characteristic of malignancy. The development of invasive carcinoma parallels development of the Müllerian duct into the epithelial tissues of the upper genital tract (Fig. 1). As the Müllerian duct is formed in the embryo from invagination of the coelomic epithelium and develops into the fallopian tube, uterine corpus and cervix, so too, does the nascent cystic ovarian neoplasm progress to its respective serous, endometrioid/clear cell and mucinous histotypes, recapitulating these Müllerian structures. An antigen derived from that Müllerian epithelium, CA-125, is a useful tumor marker for the serous carcinomas, though less so for the other histotypes.
It is of note that the endometrioid and clear cell carcinomas of the ovary have recently been identified as a separate entity based on molecular profiling. ARID1A mutations and loss of BAF250a expression in these tumor types and in associated atypical endometriotic lesions from which they originated suggest that they are a separate entity, though still arising from a variant Müllerian epithelium. These early steps in malignant transformation also suggest a potential target for future intervention.
In the strictest sense, ovarian cancer is not really cancer of the ovary. It is cancer of the epithelial surface that is derived from and contiguous with the surrounding pelvic peritoneum. Fully 10% of the patients with serous papillary carcinoma involving the pelvic viscera and omentum have disease that is histologically identical to epithelial carcinoma of the ovary and spreads in the same manner, but these patients either have no evidence of ovarian involvement or have a minor degree of involvement of the surface of the ovarian cortex. These lesions are appropriately termed serous papillary tumors of peritoneal origin. It is not surprising, therefore, that prophylactic removal of both ovaries will greatly reduce, but not eliminate all “ovarian” cancer in women with a genetic predisposition to that disease. Because of this effect, prophylactic bilateral salpingo-oophorectomy is termed a risk-reducing strategy that eliminates the source of serous papillary carcinoma from the ovary, but not from the entire peritoneal surface. One must keep this observation in mind and caution the patient that continued surveillance will be necessary.
In a similar manner, endometrioid carcinoma may arise from peritoneal surface implants of endometriosis. This can be multifocal endometrioid carcinoma involving endometriosis in the ovaries as well as foci of peritoneal endometriosis, most commonly in the cul-de-sac; at times, there may also be a second primary carcinoma in the native endometrium within the uterus. It can be a challenge for the pathologist to differentiate between multifocal primary lesions and metastatic disease from a primary endometrial carcinoma. The origin of clear cell or endometrioid carcinoma originating in endometriosis has been identified as associated with an improved prognosis probably related to increased prevalence of symptoms associated with early disease.
This parallelism indicated in Figure 1 between the embryogenesis of the upper female genital tract from the primordial coelomic epithelium and the pathogenesis of neoplasia resulting from this tissue continues to be supported by increasing molecular evidence, as noted above. Kurman, at Johns Hopkins, has proposed a further extension of this theory of pathogenesis that “type I” tumors behave in an indolent fashion and are relatively genetically stable, sharing a lineage with the corresponding benign cystic neoplasm. In addition, “type II” tumors arise from the epithelium of the fallopian tube and are highly aggressive, rapidly evolving tumors that present an advanced stage. These tumors characteristically are classified as high-grade serous carcinoma, undifferentiated carcinoma and malignant mixed mesoderm will tumors (carcinosarcoma).
It is particularly notable that even after successful primary treatment of endometrioid or clear cell carcinoma arising in endometriosis, that second primary tumors may represent a substantial proportion of what is otherwise thought to be “recurrent” carcinoma. This is particularly important for
the surgeon to recognize that late recurrence of ovarian carcinoma should be considered as potential second primary and may well represent isolated disease that can be successfully treated with surgery and potentially adjunctive chemotherapy. Future molecular characterization will better delineate this distinction in the development of second primary lesions.
the surgeon to recognize that late recurrence of ovarian carcinoma should be considered as potential second primary and may well represent isolated disease that can be successfully treated with surgery and potentially adjunctive chemotherapy. Future molecular characterization will better delineate this distinction in the development of second primary lesions.
Low-grade mucinous neoplasms of the ovary may arise from one of two origins. As is characteristic of the mucinous borderline tumors, these may be either Müllerian (and often associated with endometriosis) or an intestinal subtype. As part of the abdominal exploration of patients with mucinous ovarian tumors, it is particularly important to always evaluate the appendix as a potential primary site of disease. For reasons yet to be determined, there may be isolated and very bulky metastasis to the contralateral ovary from the appendix without evidence of intervening disease in the ipsilateral ovary or uterus. These low-grade mucinous tumors of the appendix and ovary are often the precursor to development of pseudomyxoma peritonei.
Patterns of Metastatic Disease in Ovarian Carcinoma
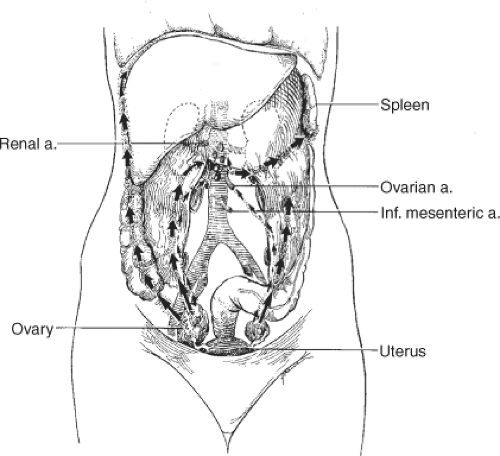
Dissemination of ovarian carcinoma from the early primary ovarian tumor predominantly occurs through transperitoneal spread (Fig. 2). Exfoliated tumor cells typically first implant in the paracolic gutters and on the right hemidiaphragm. The negative pressure associated with respiratory movement of the diaphragm over the liver creates a flow of peritoneal fluid that is absorbed at this site. Because some epithelial ovarian carcinomas actually may begin on the surface of the ovarian cortex, transperitoneal spread may occur early in the natural history of the disease. In fact, if the tumor arises as a primary peritoneal neoplasm on the surface of the pelvic peritoneum, then peritoneal spread begins de novo with an immediate transition from stage I to III disease. Implantation and growth of ovarian carcinoma on the diaphragm leads to the obstruction of lymphatic flow and contributes to the accumulation of ascites. That process is also facilitated by the influence of VEGF production by the ovarian malignancy as part of the process of angiogenesis. Among patients with intestinal carcinomatosis, a common site of tumor implantation is along the junction of the mesentery and the bowel wall where blood vessels may be most accessible to tumor angiogenesis (Fig. 3).
Poorly differentiated tumors also commonly spread along ovarian lymphatics to regional nodes at the para-aortic level as well as to pelvic nodes. Patients with high-grade primary ovarian tumors clinically confined to the ovary may have evidence of occult regional spread to the pelvic nodes as well as to para-aortic nodes at the origin of the gonadal arteries. These lymph node metastases may be the only site of metastatic disease. Patients with predominately lymph node metastases in the absence of transperitoneal spread actually have a better prognosis than patients with negative nodes and intraperitoneal disease.
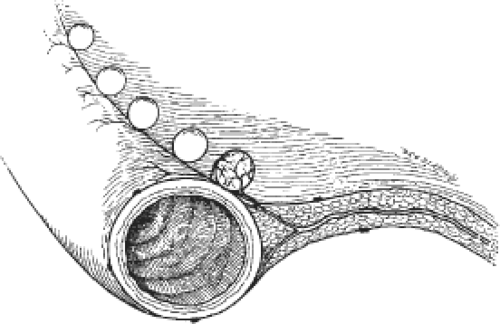 Fig. 3. Growth of tumor occurs along the interface between mesentery and bowel wall where the vascular supply is exposed to tumor angiogenesis up. |
The pattern of lymph node metastasis in patients with stage III (abdominal spread) ovarian cancer and any nodal metastasis is such that the majority of patients will have both pelvic and para-aortic nodal diseases, while a minority will have either pelvic or para-aortic disease. Patients with primary peritoneal cancer with disease not extensively involving the ovary have a lower incidence of nodal metastases for the same extent of intra-abdominal disease.
Early transperitoneal spread of ovarian carcinoma is classically identified in the right paracolic gutter and on the right hemidiaphragm. Several typical patterns of spread are identified with more advanced disease: some patients may present with disease solely in the omentum, some with “oligo” metastatic disease (bulky metastases, few in number, usually in the omentum and on peritoneal surfaces), and some with miliary carcinomatosis. Obviously each of these patterns has implications for the surgeon as it affects the resectability of this tumor and intraoperative planning of the sequence of tumor resection. Because stage II disease is relatively uncommon in light of what we understand about peritoneal spread, its occurrence is typically associated with bulky, low-grade tumors and direct rectosigmoid invasion. The association of endometrioid carcinoma and endometriosis accounts for a number of these patients, who, by definition, have local peritoneal involvement, arising in pelvic endometriosis. The locally invasive nature of this tumor is also consistent with Kurman’s view of these lesions as “type I” lesions, less likely to be associated with extrapelvic metastatic disease. This locally advanced disease can be treated successfully with en bloc resection of the ovarian or peritoneal neoplasm, the uterus, pelvic peritoneum, and the rectosigmoid colon.
The predisposition for this disease to be multifocal and arising in endometriosis also accounts for the problem of local recurrence in pelvic peritoneum. Presumably many cases of pelvic recurrence may represent a field effect with new cancers arising
in residual pelvic endometriosis. The long-term management of epithelial carcinoma arising in endometriosis does represent an indication for postoperative pelvic radiation therapy to achieve a durable prolonged remission. The identification of second primary tumors in these patients has substantial implications for the use of secondary cytoreductive surgery for what is apparently recurrent disease. In similar manner, as noted above, the pattern of ovarian cancer spread to lymph nodes exclusively appears to carry a better prognosis, as the absence of associated peritoneal metastases seems to reduce the risk of peritoneal recurrence.
in residual pelvic endometriosis. The long-term management of epithelial carcinoma arising in endometriosis does represent an indication for postoperative pelvic radiation therapy to achieve a durable prolonged remission. The identification of second primary tumors in these patients has substantial implications for the use of secondary cytoreductive surgery for what is apparently recurrent disease. In similar manner, as noted above, the pattern of ovarian cancer spread to lymph nodes exclusively appears to carry a better prognosis, as the absence of associated peritoneal metastases seems to reduce the risk of peritoneal recurrence.
The clinical presentation at the time of diagnosis also may reflect the distribution of tumor, the tumor grade, and by inference, the patient’s prognosis. Patients presenting with a typical picture of abdominal distention and symptoms of bowel obstruction most commonly have fairly extensive peritoneal carcinomatosis. On the contrary, patients with the largest pelvic masses often have compressive symptoms secondary to tumor bulk without extensive invasion or metastasis; hence, in the absence of invasion, the tumor has sufficient time to grow to large size. Some patients with surgical stage IV disease actually have an excellent prognosis, if they present solely with femoral nodal metastases, as may be characteristic of patients with a bulky adnexal mass associated with serous papillary carcinoma of the fallopian tube.
We have also observed that patients with stage IV disease on the basis of pleural effusion without a radiographically identifiable mass in the chest will have an outcome that is no different from that of a patient with comparable stage III disease. On that basis, it may be appropriate in a future revision of the International Federation of Obstetrics and Gynecology (FIGO) staging system to consider pleural fluid only stage IVA disease, distinct from parenchymal organ involvement or distant nodal involvement as stage IVB disease. The surgeon should not be dissuaded from consideration of a surgical approach to this disease, although it may be preferable to consider thoracentesis and a single cycle of intravenous chemotherapy prior to surgery as this will prevent both intraoperative and postoperative pulmonary complications secondary to re-accumulation of that malignant pleural effusion.
Given what we know about the pathogenesis of ovarian and peritoneal carcinoma and the propensity for early transcoelomic spread, the extreme difficulty of early diagnosis may be better understood. Clearly, for patients with primary peritoneal neoplasms, there is no “stage I” disease, as peritoneal spread is part of the definitive histogenesis. The paucity and lack of specificity of symptoms associated with early primary ovarian cancer presents additional difficulties in diagnosis. It is not until the disease has extended to the gastrointestinal tract, or has metastasized to the omentum and produced ascites, that clinical symptoms become evident. Even at this point, there are nonspecific gastrointestinal symptoms, typically bloating or vague crampy abdominal pain, that are attributed to three prevalent gastrointestinal disorders: gallbladder disease, hiatus hernia, and diverticular disease. An abnormal CA-125 assay, though not always present, will help the general surgeon avoid exploration of the patient for a radiographically identifiable lesion, only to find stage III metastatic ovarian carcinoma.
In similar manner, research in screening for ovarian cancer has not yet produced a useful diagnostic algorithm. The techniques available today include a review of clinical symptoms, physical examination, pelvic ultrasound, and the CA-125 assay as mentioned above. The low prevalence of clinically occult disease in young, premenopausal women (1:3,000) and the relatively high frequency of false-positive testing with CA-125 and pelvic ultrasound make routine screening inadvisable in this age group. Screening low risk premenopausal women, in the absence of a strong family history will produce a ratio of false positive to true positive testing of about 200:1. In the postmenopausal woman, a greater prevalence of occult disease (1:1,000) and the greater specificity of the screening tests improve their value, but still only bring it down to 30 to 1, not sufficient for routine screening.
In a patient with a pelvic mass detected on routine clinical examination, or as a result of radiographic investigation of clinical symptoms, the adjunctive use of a CA-125 assay will help to predict the presence of benign versus malignant disease. One must be mindful of the prevalence of low levels of elevated CA-125 in premenopausal women associated with benign disease such as fibroids and pelvic endometriosis. Typically, the CA-125 level will be elevated to no more than 200 or 300. A higher level makes the diagnosis of malignancy more likely. A recent proteomics approach that involves measurement of a number of tumor associated proteins as well as CA-125 does appear to have improved specificity in evaluation of patients with a pelvic mass. In any case, however, the presence of a complex ovarian mass is an indication for surgery, while some simple cysts, particularly in postmenopausal women, can be followed conservatively.
Prophylactic Surgery for Women At High Risk
Risk reducing oophorectomy for the patient with an elevated risk of ovarian carcinoma may take two forms: the prevention of disease with incidental bilateral oophorectomy at the time of other elective pelvic or abdominal surgery or elective laparoscopic bilateral oophorectomy. In evaluation of the patient with a positive family history of breast and ovarian carcinoma (hereditary breast and ovarian cancer [HBOC] syndrome), one takes into account the age at which the disease develops in the index case(s) as well as the closeness of that relationship to the patient at risk. In the general population, the cumulative risk of developing ovarian cancer is 1.5% (1:70) with a 1% lifetime risk of death from the disease. Relatives with premenopausal HBOC are more likely than those with postmenopausal recurrence of disease to bear a predisposing deleterious mutation. A single first-degree relative confers as much as a fivefold greater risk, while two first-degree relatives with premenopausal disease may increase it to 50%. The association of premenopausal breast and ovarian carcinoma recognized in association with a deleterious BRCA1 or BRCA2 mutation may present as much as a 50% risk of subsequent ovarian carcinoma. Recent analysis of HBOC patients without BRCA1 and BRCA2 mutations has demonstrated that 61% of these patients had a variant allele of KRAS. This KRAS-variant represents a new marker for inherited cancer risk in HBOC families.
Laparoscopic oophorectomy can be easily performed as an outpatient procedure and will reduce, but not eliminate the risk of subsequent “ovarian” carcinoma, as it does not diminish the incidence of primary peritoneal carcinoma. Prior to the time at which prophylactic oophorectomy may be appropriate, the young woman at risk will benefit from the use of ovulation suppression with oral contraceptive therapy. It is in these patients where the prevalence of subclinical ovarian carcinoma is greater, that routine screening with pelvic ultrasound and CA-125 may be appropriate.
Incidental oophorectomy should be performed at the time of other pelvic surgery in the postmenopausal woman. The striking increase in incidence of ovarian carcinoma after menopause, coupled simultaneously
with the decreasing endocrine function of the ovary, makes risk–benefit profile of incidental oophorectomy increasingly favorable with advancing age. As women become cognizant of the difficulty in screening for ovarian malignancy, the surgeon should discuss this option in women undergoing elective pelvic or lower abdominal procedures. Following oophorectomy, patients who have no contraindications to estrogen therapy and have an intact uterus will generally receive short-term course of daily estrogen and progesterone. The progesterone is added to minimize the risk of subsequent endometrial neoplasia and is not needed in the absence of the uterus or active endometriosis.
with the decreasing endocrine function of the ovary, makes risk–benefit profile of incidental oophorectomy increasingly favorable with advancing age. As women become cognizant of the difficulty in screening for ovarian malignancy, the surgeon should discuss this option in women undergoing elective pelvic or lower abdominal procedures. Following oophorectomy, patients who have no contraindications to estrogen therapy and have an intact uterus will generally receive short-term course of daily estrogen and progesterone. The progesterone is added to minimize the risk of subsequent endometrial neoplasia and is not needed in the absence of the uterus or active endometriosis.
Diagnostic Management of the Clinically Localized Ovarian Mass
The evaluation of the pelvic mass in a young premenopausal woman should address both the differential diagnosis that includes assessment risk of malignancy and the possibility of its resection by laparoscopy. Indeed, there are few limitations to laparoscopic surgery: those would include masses that are fixed in the pelvis, typically associated with endometriosis and masses that are so large as to preclude laparoscopic removal without drainage or rupture. Even these limitations are relative, in the sense that fixed masses can often be dissected from the pelvic sidewall with the attached pelvic peritoneum and large cystic masses that have a simple internal architecture may be carefully drained in some limited circumstances where the risk of malignancy is very small.
Preoperative assessment of the patient with a presumed ovarian mass who is considered a candidate for laparoscopic oophorectomy or cystectomy should include the following:
Pelvic examination with the particular attention to both the mobility of the mass, including fixation to the pelvic sidewall, and the isolated nature of that lesion—that there are no pelvic peritoneal nodules or irregular cul-de-sac surfaces that would suggest the possibility of occult metastatic disease. However, the problem of distinguishing neoplastic nodules in the paracervical soft tissue from implants of endometriosis in patients at risk for that disease is difficult. The mass should not be fixed to the pelvic sidewall, making successful laparoscopic resection without rupture unlikely.
A preoperative CA-125 or OVA1 level that should be within the range of normal; mildly elevated levels should raise suspicion of occult malignancy, but should not be an absolute contraindication to laparoscopic surgery. As indicated above, in premenopausal patients, moderate elevations of CA-125 up to the 200 range, or more, can be expected with uterine fibroids or pelvic endometriosis; values over the 300 range should raise suspicion of malignancy. Conversely, ovarian cancers may also arise and may metastasize in the presence of a normal or minimally elevated CA-125 values as well—commonly with clear cell/endometrioid and mucinous epithelial tumors. Most germ cell or gonadal stromal tumors will present as relatively solid, sometimes centrally necrotic tumors and not be associated with an elevated CA-125 unless there is peritoneal involvement by surface metastases. In the young woman at risk for germ cell or stromal cell neoplasia, relevant biomarkers (hCG, alpha-fetoprotein, inhibition B, MIS, LDH) need not be obtained until the postoperative period when the diagnosis is more likely or certain, unless such knowledge would influence the decision for referral to a gynecologic oncologist. The most conservative procedure compatible with obtaining a diagnosis in the circumstances is appropriate.
Transvaginal or transabdominal pelvic ultrasound that can evaluate the internal architecture of the ovary, assess the status of the contralateral ovary and identify any evidence of metastatic disease, including ascites, that might indicate the need for an open procedure. At times, use of magnetic resonance imaging (MRI) will be helpful in that endometriomas contain degraded blood products and yield a relatively homogeneous high signal intensity on T1-weighted images and relatively hypo-intense signal on T2-weighted images. Evidence for a large solid component should also raise the question of occult malignancy, unless the mass has the extremely hard consistency characteristic of an ovarian fibroma on clinical examination. A pedunculated fibroid may be another source of solid adnexal mass and if solitary, may require a pelvic MRI to make an accurate diagnosis without laparoscopy.
Laparoscopic Management of the Ovarian Neoplasm
The mobility of the mass at the time of examination under anesthesia may be the strongest predictor of operability. Otherwise, in a premenopausal woman, the obvious laparoscopic appearance of endometriosis may be the most useful intraoperative finding that would suggest that one should proceed with surgical resection in the absence of a freely mobile mass. Biopsy of individual serosal implants for frozen section examination will be reassuring in that regard.
In a young woman with apparent benign disease, a laparoscopic ovarian cystectomy with preservation of the integrity of the majority of ovarian tissue would be the appropriate conservative course of action. Ideally, the cyst should be removed and placed intact into a laparoscopic bag for removal. Typically, one will find residual ovarian tissue to be preserved along the ovarian vessels, though occasionally tumors of very large size do not demonstrate any recognizable residual normal ovarian cortex.
In postmenopausal women where the risk of occult malignancy is greater and the value of preservation of one ovary is lesser, the appropriate course of action is to perform an oophorectomy rather than an ovarian cystectomy, particularly if there is any suspicious appearance to the ovarian mass. Generally speaking, any major degree of fixation of the ovarian neoplasm in the absence of obvious endometriosis should both raise the suspicion of malignancy and increase the likelihood of cyst rupture at the time of laparoscopic intervention.
One of our previous publications has addressed the clinical significance of cyst rupture on prognosis and supports the concept that the measurable adverse effect of cyst rupture may well be related to a delay in intervention after the event occurs. Alternatively, the presence of occult invasion of adjacent soft tissues may account for adhesion and rupture. Hence, more advanced disease may have been present than clinically recognized; cyst rupture then representing a manifestation of extra-ovarian spread and potential residual locally metastatic disease at the site of rupture. Immediate intervention and wide excision of surrounding soft tissue in the presence of rupture of a potentially malignant cyst, proven by frozen section, might be considered reasonable on that basis. However, as most of these patients now would be treated with chemotherapy in that event, the need for immediate surgical intervention is limited to being certain that all potential local sites of extension are excised. Assessment of occult distant spread is better handled with complete surgical staging in the hands of someone skilled in that procedure.
Surgery for Localized Ovarian Carcinoma
Patients with early invasive cancer diagnosed at the time of laparoscopy or laparotomy
require the same intensive staging procedure (Table 1) irrespective of the route of access. In the absence of overt metastatic disease, a minimally invasive approach to surgical staging can be considered, either laparoscopically or with a robotic-assisted approach. The patient should undergo a preoperative mechanical bowel prep, if only to facilitate visualization of the pelvic and abdominal viscera. The surgeon should be prepared to carry out a definitive staging and carefully examine (but not necessarily biopsy) the contralateral ovary as well, particularly if conservative management is warranted. The consistent difficulty for laparoscopic or robotic-assisted staging is gaining access to para-aortic nodes at the level of the renal vein and particularly those nodes that lie posterior to the left renal vein.
require the same intensive staging procedure (Table 1) irrespective of the route of access. In the absence of overt metastatic disease, a minimally invasive approach to surgical staging can be considered, either laparoscopically or with a robotic-assisted approach. The patient should undergo a preoperative mechanical bowel prep, if only to facilitate visualization of the pelvic and abdominal viscera. The surgeon should be prepared to carry out a definitive staging and carefully examine (but not necessarily biopsy) the contralateral ovary as well, particularly if conservative management is warranted. The consistent difficulty for laparoscopic or robotic-assisted staging is gaining access to para-aortic nodes at the level of the renal vein and particularly those nodes that lie posterior to the left renal vein.
Table 1 The FIGO Staging System for Ovarian Carcinoma | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Some gynecologic oncologists still prefer an open approach to these nodes for the patient with a poorly differentiated ovarian lesion; others feel that if one is truly going to treat with chemotherapy on the basis of a high-grade primary lesion alone, that resection of nodes appearing normal on computed tomography (CT) scan or at surgical exploration be unnecessary. This latter view remains controversial for patients advanced disease, though not so for patients with limited ovarian disease. Analysis from the SEER database in the United States indicates inferior survival among those patients who do not undergo systematic lymphadenectomy. A multicenter review of patients with extensively staged localized disease further supports this approach with an excellent outcome among patients undergoing comprehensive surgical staging. In a prospective European trial for advanced ovarian cancer, there was no difference in long-term outcome between patients having selective removal of enlarged pelvic and abdominal lymph nodes and those having complete pelvic and para-aortic lymphadenectomy. Clearly, however, it was important to remove any macroscopically enlarged lymph node, if that represented the largest site of residual disease.
Conventional wisdom dictates that, even in young women, the use of a vertical midline or paramedian incision should be a prerequisite for the adequate staging of a patient with possible ovarian carcinoma, to explore diaphragmatic surfaces, biopsy suspicious upper abdominal lesions, and carry out para-aortic node dissection. However, with healthy, slender young women, consideration of a more cosmetically favorable transverse incision frequently arises in patients at relatively low risk for invasive carcinoma. My personal preference is not to pursue that conventional approach for such patients, but to be conservative in the choice of incision.
Use laparoscopy for small lesions that can be removed with this minimally invasive approach, placing the ovarian tumor in a polyethylene or nylon bag for extraction. When the bag opening is pulled through the abdominal wall, specimen retrieval is carried out through an extracorporeal route, by morcellation, if necessary.
Consider conversion to laparotomy with a vertical incision, if there is evidence of metastatic disease that is not easily resectable, along with complete surgical staging.
Consider robotic surgery for a fixed mass in the young woman for whom the diagnosis may well be pelvic endometriosis if it is not amenable to laparoscopy. If this is not possible then primary laparoscopy with possible conversion to a Pfannenstiel incision for a benign lesion that requires open surgery for resection would be appropriate.
Consider ovarian cystectomy for a lesion that appears smooth surfaced and unilocular in the young women who wishes to preserve reproductive and/or endocrine function.
Lesions that are large or fixed in nature are probably best be treated by an open approach, as are large tumors associated with an elevated CA-125 level in the postmenopausal woman.
With limited pelvic disease in a postmenopausal patient, robotic surgery with preliminary laparoscopy to evaluate the upper abdomen provides a minimally invasive option that also permits extensive surgical resection from the pelvis to the midabdomen. In the presence of upper abdominal disease identified at laparoscopy, one can easily convert to a laparotomy through a vertical incision.
With the paramedian incision, one has a simple linear incision quite near the midline; one is not limited to keep the incision below the umbilicus without hooking around its contour. The fascial incision may even be extended obliquely to the linea alba.
If one does perform a laparoscopy and finds evidence of what appears to be a malignant neoplasm in a young woman, particularly if it is clinically localized to
the ovary, it is always wise to be certain of the diagnosis on permanent histopathology before consideration of extensive surgical therapy that might impair future fertility. One can, and often should, go back at a later date when the patient is fully informed and better prepared to undergo major surgery. This is obviously much easier to do laparoscopically than with an open abdominal incision.
Tumor resection and extent of disease evaluation are two components of the surgical procedure that are integral to management of the patient with an apparently localized ovarian lesion. Staging is carried out in accordance with the protocol delineated by the FIGO and requires the following:
Table 2 Criteria for Conservative Management of Localized Ovarian Malignancy | ||
|---|---|---|
|
Assessment of the cytology of any pelvic fluid or washings of the pelvic viscera to identify free cells within the peritoneal cavity.
Biopsy of any areas of adherence of the ovarian tumor in the pelvis or of any areas of contiguous spread of the tumor within the pelvis.
Evaluation of pelvic nodes, ideally with systematic lymphadenectomy in early-stage disease and at least with resection of all enlarged nodes in patients with advanced disease whose intraperitoneal component has been resected. In the absence of any other sites of extra-ovarian spread, a complete systematic node dissection is necessary to completely evaluate the risk of metastasis as well is to remove residual disease. If there is histologic evidence of extensive metastatic intraperitoneal disease, then sampling of nodes and only removal of all enlarged nodes may be sufficient, although this is somewhat controversial, as noted from the SEER data.
Examination of the upper abdomen, with resection of the omentum, and evaluation of the paracolic gutters and the diaphragms. It is particularly important to evaluate the posterior aspect of the diaphragm behind the liver as well as the inferior aspect of the liver at the foramen of Winslow.
Resection of the para-aortic lymph nodes at the level of the origin of the ovarian vessels. Again, in the presence of metastatic carcinoma elsewhere, resection of only those enlarged nodes containing metastatic disease may be all that is necessary.
This comprehensive staging procedure is necessary for the accurate assessment of risk for recurrence and the need for postoperative chemotherapy. Essentially all patients with disease involving the ovarian surface and beyond are candidates for chemotherapy. Patients with high-grade lesions confined to the ovary or those with less aggressive lesions who are found to have cyst rupture, particularly if associated with dense pelvic adhesions, are also candidates for chemotherapy. Alternatively, some young women of childbearing age may undergo conservative therapy with preservation of reproductive function (Table 2). The primary tumor must be of low histologic grade and rigorously staged. These patients should undergo completion of the definitive surgical procedure following conclusion of childbearing.
At the present time, adjuvant chemotherapy has no role in treatment of well-differentiated early invasive epithelial malignancies confined to the ovary with no surface involvement. The data regarding grade 2 tumors do not clearly indicate a benefit for adjuvant chemotherapy and only with grade 3, stage IA tumors as well as for more advanced lesions, is there a role for adjuvant treatment. As the success with pregnancy following chemotherapy of other malignancies is well supported in the literature, it is reasonable to consider this possibility in carcinoma of the ovary as well. Patients with stage IA, G3 and all with unilateral stage IC disease should receive adjuvant platinum and taxane-based chemotherapy along with preservation of a normal contralateral ovary. Patients with stage IB disease, or more advanced disease with contralateral ovarian involvement may require bilateral oophorectomy. A careful deliberation with the patient and her family and clearly defined informed consent is critical to this course.
Special Case of Ovarian Tumors of Borderline Malignancy
In contrast to the fully malignant epithelial tumors of the ovary, conservative surgical management of ovarian tumors of borderline malignancy should be considered the rule for young women, rather than the exception. Faced with an ovarian neoplasm with surface excrescences and implants within the peritoneal cavity and omentum, if the histologic diagnosis suggests an ovarian tumor of borderline malignancy, one should still consider the possibility of preservation of reproductive potential, even if the excision of all of one ovary and part of another might be required for removal of apparent malignant disease. The difficulty in distinguishing between invasive tumors and tumors of borderline malignancy on frozen section evaluation, particularly with limited sampling of bulky tumors, is the basis for the general recommendation that one should never ablate reproductive function in a young woman of childbearing age on the basis of frozen section alone.
Controversial issues related to the surgical care of the patient with a borderline ovarian tumor include the following:
Cystectomy versus oophorectomy in the management of the primary tumor—particularly as many tumors will only be
definitively diagnosed by histopathology after laparoscopic ovarian cystectomy.
The need to biopsy the opposite, often normal appearing—ovary; one must balance the yield from the procedure versus the risk of infertility, which is usually already an issue by that time.
The need to fulfill the requirements of FIGO staging, with pelvic and para-aortic node dissection in particular, when no definitive therapy is usually recommended for patients with nodal “metastases” that may actually represent Müllerian inclusions and not metastases from a noninvasive lesion.
Management of patients with peritoneal implants also presents a difficult problem. Implants may be noninvasive, desmoplastic, or invasive. Each carries a different prognosis and prudent management demands adequate sampling of a substantial proportion, if not all, of the lesions identified. Complete removal of all macroscopic lesions should be the goal—both to excise the tumor itself and also to ensure that a more aggressive metastatic lesion is not missed. Only a small proportion of women with borderline tumors will have invasive implants and present a risk of recurrence and death.
Monitoring of the typical patient with noninvasive implants, the patient with a stage III tumor of borderline malignancy, requires regular pelvic examinations and quarterly CA-125s for the first year. After that time, progressively longer follow-up intervals would be appropriate. Routine CT scans in the absence of symptoms or a rising CA-125, even for nonserous tumors, would not appear to be indicated.
Chemotherapy for these patients with borderline tumors and advanced or recurrent disease is unlikely to produce any dramatic and sustained responses because of the low-grade nature of these tumors, their functional similarity to the corresponding normal tissue, and the low growth fraction assayed in vitro. There is some evidence, in fact, that chemotherapy may actually induce more aggressive behavior—presumably causing further malignant progression with dedifferentiation as a consequence of the mutagenic effects of the chemotherapeutic agents. Hope in the future for biologic agents that will promote (re)differentiation in these minimally deviant tumors may represent the best opportunity for disease control.
Surgery for Advanced Ovarian Carcinoma: Overview
At the time of diagnosis, ovarian carcinoma presents with the greatest tumor burden of any solid tumor; a kilogram or more of tumor may be distributed in the pelvis and upper abdomen, along with several liters of ascites. Although the epithelial ovarian tumors are considered quite responsive to primary chemotherapy with response rates in the 80% to 90% range in untreated individuals, the dominant variable predictive of long-term survival is the amount of residual disease present at the conclusion of the initial surgical procedure. The validity of less than total excision in the presence of obvious metastatic disease appears to violate basic tenets of surgical oncology and has often been questioned. Nonetheless, it has been subjected to careful scrutiny over the last three decades and has been consistently documented as the dominant prognostic factor in studies of patient survival, whether treatment involves chemotherapy or radiation therapy. Essentially, every clinical study has documented that the extent of residual disease even outweighs surgical stage in its prognostic importance.
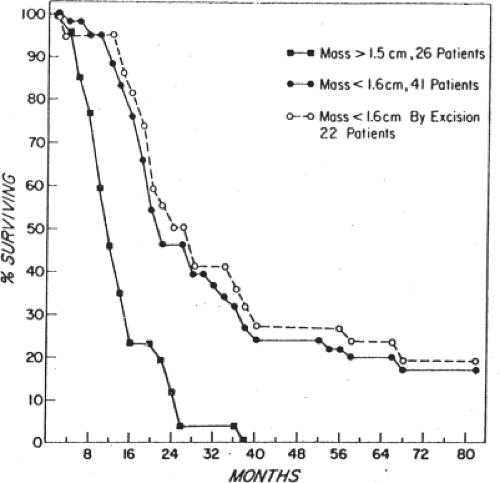
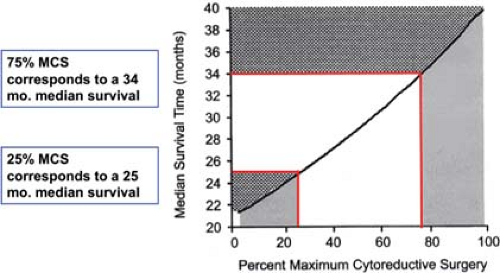
Griffiths, in an NCI monograph, demonstrated that patients with bulky intra-abdominal spread of ovarian cancer who underwent extensive cytoreduction to minimal residual disease had the same median survival time as those patients who had the same amount of minimal residual disease, but did not require extensive surgical cytoreduction. We have similarly examined the outcome of patients requiring multiple resections at the time of primary surgery, and have contrasted that with the outcome of patients requiring much more limited resection (Fig. 4). As long as the amount of residual disease remained the same at the conclusion of surgery, there was no difference in survival, whether the patient had a simple hysterectomy, bilateral salpingo oophorectomy and gastrocolic omentectomy, or, in addition, splenectomy, transverse colon resection, rectosigmoid resection, small bowel resection and resection of enlarged pelvic and para-aortic nodes. The theoretical explanation employed to support this observation is that surgical cytoreduction removes bulky tumor that is either constitutively drug resistant, or one that is resistant because of poor drug penetration into a large tumor mass. In addition to this effect, reduction from a 5-cm tumor mass to a 0.5-mm tumor mass represents a seven log tumor cell reduction, from 1011 cells to 104 cells, decreasing the amount of chemotherapy needed for tumor control based on first-order kinetics of cell kill. This threshold effect may further be explained in theory by the elimination of cells that carry extreme drug resistance (Fig. 5).
There is a threshold, however, above which surgical resection does not incrementally improve survival; that appears to be a largest residual tumor mass of 1.0 to 1.5 cm. Resection of disease to that level, or below does improve survival time and there is a further increment in survival with every additional decrement in residual disease, down to the microscopic level. Traditionally, resection to a largest residual mass of
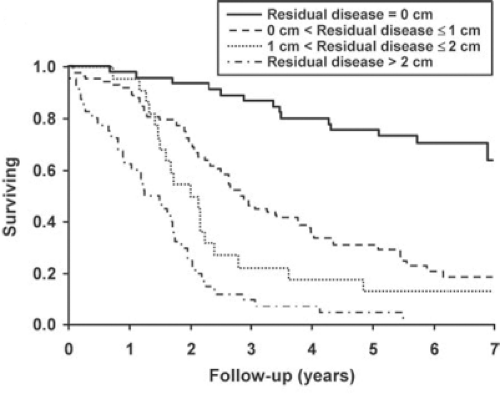
1.5 cm or less has been termed “optimal” cytoreduction, in the sense that resection to this level or below to improves survival. As the Latin root for “optimal” is “best,” one should reserve that term for complete cytoreduction. The appropriate term for cytoreduction to the threshold level or below is “adequate,” as it is adequate for improvement in survival. In common use, however, the reasonable compromise is to call complete cytoreductive surgery “complete,” anything else is “incomplete,” either optimal, or suboptimal. As noted in Figure 6, patients who underwent complete cytoreduction had a 5-year survival in excess of 75%, while patients who had macroscopic residual disease, but <1 cm in size had about a 30% survival and patients with residual disease >1 cm but <2 cm had only a 15% 5-year survival. This retrospective study from the Mayo Clinic underscores the primacy of complete cytoreductive surgery in the surgical management of this disease.
1.5 cm or less has been termed “optimal” cytoreduction, in the sense that resection to this level or below to improves survival. As the Latin root for “optimal” is “best,” one should reserve that term for complete cytoreduction. The appropriate term for cytoreduction to the threshold level or below is “adequate,” as it is adequate for improvement in survival. In common use, however, the reasonable compromise is to call complete cytoreductive surgery “complete,” anything else is “incomplete,” either optimal, or suboptimal. As noted in Figure 6, patients who underwent complete cytoreduction had a 5-year survival in excess of 75%, while patients who had macroscopic residual disease, but <1 cm in size had about a 30% survival and patients with residual disease >1 cm but <2 cm had only a 15% 5-year survival. This retrospective study from the Mayo Clinic underscores the primacy of complete cytoreductive surgery in the surgical management of this disease.
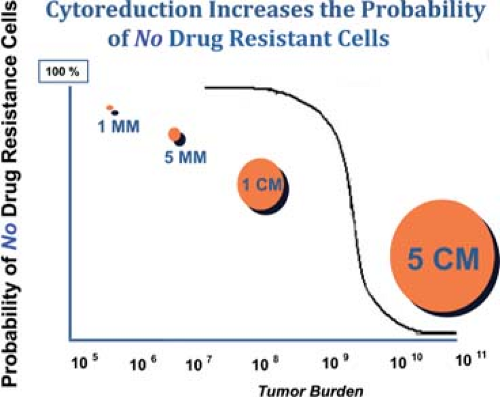 Fig. 5. Cytoreductive surgery below a threshold, as indicated, eliminates the very small proportion of cells that may have de novo extreme drug resistance. |
The success of cytoreductive surgery is dependent upon the metastatic pattern of the tumor spread as well as the experience of the surgeon. Rarely is the pelvis the site of residual disease after incomplete cytoreduction. The challenge to the gynecologic oncologist is the resection of metastases to the upper abdomen. Common sites of surgical failure are the root of the small bowel mesentery, nodal disease above the celiac axis involving the lesser curve of stomach and the left lobe of liver, and perihepatic disease on the right as well as peripancreatic disease involving the C-loop of the duodenum. Although there may be an element of patient selection for surgical exploration, the proportion of patients who undergo “adequate” cytoreductive surgery should be in the 80% to 90% range; approximately half of these patients should have no more than 2 to 3 mm residual disease. Bristow has demonstrated that institutions with a high-frequency of optimal cytoreduction also have improved survival when compared with institutions that do not have such a level of surgical success (Fig. 7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree