Surgical Anatomy of the Pancreas
David A. Kooby
Marios Loukas
Lee J. Skandalakis
David A. McClusky III
Petros Mirilas
Introduction
The pancreas is a bland-appearing yellowish organ situated in the retroperitoneum. Early historical descriptions of this gland are hardly more than a list of names of those who noticed it during their dissections, before moving on to more interesting organs. It was only with identification of the digestive enzymes by Claude Bernard in 1850 that the pancreas became recognized as a complete organ with an important function, and, thus, worthy of study.
The retroperitoneal location and anatomic relationships of the pancreas combine to make its surgical removal difficult. Sir Andrew Watt Kay wrote in 1978, “For me, the tiger country is removal of the pancreas. The anatomy is very complex and one encounters anomalies.” The embryogenesis of the pancreas and its deep retroperitoneal anatomy are responsible for the designation “tiger country.”
Prior to exploration, the surgeon should know whether the patient’s pancreas contains any anomalies of the parenchyma (annular pancreas or pancreas divisum), ductal system (pancreas divisum, obstruction, or aberrant location), or vasculature (replaced right hepatic artery behind the pancreatic head or other arterial anomalies). At operation, the surgeon must consider these possibilities, even if they are not evident on preoperative imaging. Furthermore, the pancreas is surrounded by numerous structures (i.e., the duodenum, stomach, spleen, left adrenal gland, transverse mesocolon and colon, left kidney, right ureter, and jejunum), making dissection of and around the pancreas potentially risky to multiple organs.
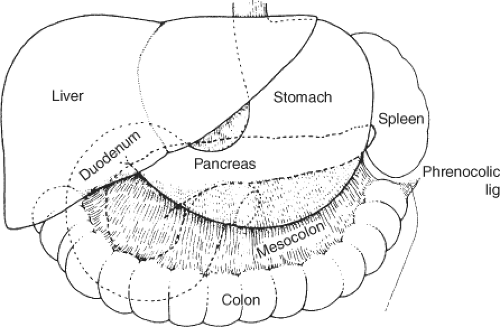
The pancreas lies transversely in the retroperitoneal space, between the duodenum on the right and the spleen on the left. It is related anteriorly to the stomach, to the omental bursa above, the greater sac below, as well as to the transverse mesocolon. It is a fixed organ. Figures 1 to 3 demonstrate the relationships of the pancreas and surrounding organs.
Embryology
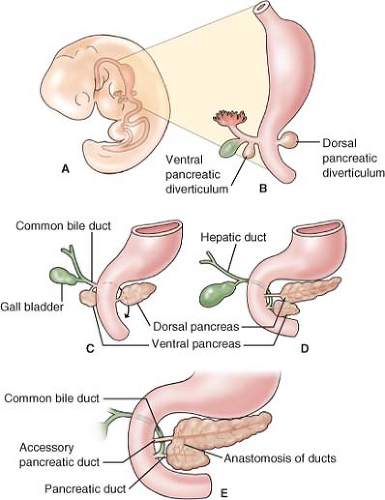
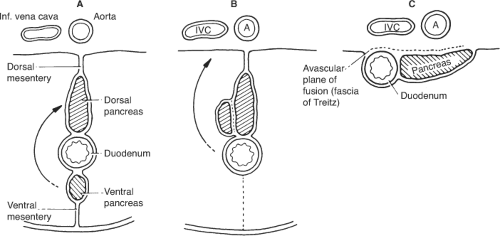
The pancreas develops from two buds originating from two endodermal primordia: the dorsal diverticulum and the ventral diverticulum of the foregut (Fig. 4A,B). The ventral pancreas develops at 4.5th to 5th week from the hepatic diverticulum, which also gives genesis to the gallbladder and the extrahepatic biliary ducts. The ventral pancreas is developmentally associated with the bile duct. The dorsal pancreas arises a bit earlier (fourth week) in the mesentery of the duodenum (mesoduodenum) and has its own pancreatic duct.
With growth and development of the bile duct the ventral pancreas is separated from the duodenum and the respective pancreatic duct exits at the common junction with the bile duct (Fig. 4C). “Rotation” of the duodenum brings the ventral pancreas and pancreatic duct–bile duct junction in the mesoduodenum to a caudal and posterior position to the dorsal pancreas (Fig. 4D). When the two pancreatic primordia come into contact they fuse (end of sixth week). The ventral primordium forms the uncinate process and the posterior head of the pancreas. The anterior head, the body, and the tail are derived from the dorsal primordium.
The ducts follow the fusion of pancreatic primordia and join. The ventral duct usually persists and unites with the distal part of the dorsal pancreas to form the main pancreatic duct. The proximal part of the dorsal pancreas either persists and forms the accessory pancreatic duct, or disappears (Fig. 4E).
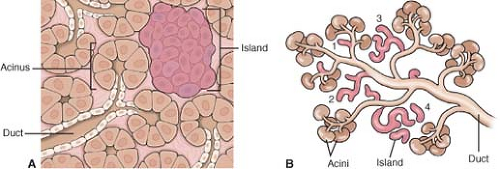
The secretory acini derive from the ducts (3rd month) and appear as clusters of cells around the duct endings. The acini secrete pancreatic enzymes and are responsible for the exocrine pancreatic function. The pancreatic islets are endocrine cells; they also originate from the terminal ducts. The islets organize into islands (Fig. 5), which comprise endocrine cells of various cell types.
Each cell type secretes a different hormone, including glucagon (A cells), insulin (B cells), and somatostatin (D cells). In addition to the acinar and endocrine cells, the terminal ducts also give rise to the ductal epithelium.
Each cell type secretes a different hormone, including glucagon (A cells), insulin (B cells), and somatostatin (D cells). In addition to the acinar and endocrine cells, the terminal ducts also give rise to the ductal epithelium.
 Fig. 2. Posterior relationships. (From Skandalakis JE, Gray SW, Rowe JS Jr., et al. Anatomical complications of pancreatic surgery. Contemp Surg 1979;15:17.) |
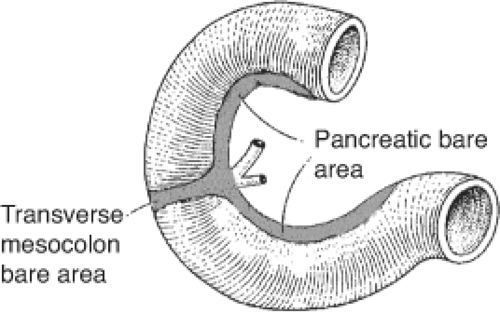
The development of the omental bursa coincides with repositioning of the viscera of the upper abdomen (see Chapter 80 “Anatomic Considerations of Gastroduodenal Surgery”). The dorsal mesogastrium bends to the right, resulting in positioning the pancreas in contact with the posterior abdominal wall (Fig. 6). The pancreas and the second portion of the duodenum become retroperitoneal organs. The peritoneum of the right side of the pancreas fuses with the parietal peritoneum of the posterior abdominal wall, creating an avascular plane called the fascia of Treitz. It is this plane that permits surgical mobilization of the gland at the time of surgical exploration.
Congenital Anomalies
Agenesia–aplasia–hypoplasia: Aplasia means failure of development; agenesia is failure to form. With respect to pancreatic primordia, both conditions result in growth retardation and early death. Hypoplasia means incomplete development and is often compatible with life. There are case reports of absence of portions of dorsal pancreatic origin, including the distal body and tail. The uncinate process is absent in 10% of pancreatic head specimens.
Hyperplasia–hypertrophia: Hyperplasia is the increase in cell numbers and hypertrophia is the growth in volume and size of existing cells. The Beckwith–Wiedemann syndrome (neonates with increased body weight, macroglossia, and omphalocele) may manifest with islet cell hypertrophy and hyperplasia with resultant hyperinsulinemia
and severe hypoglycemia. For the most part, treatment is conservative; however, partial pancreatectomy may be necessary in cases refractory to medical therapy.
and severe hypoglycemia. For the most part, treatment is conservative; however, partial pancreatectomy may be necessary in cases refractory to medical therapy.
Pancreas divisum (isolated ventral pancreas): Pancreas divisum represents failure of the ventral and dorsal pancreas to fuse, resulting in the main and the accessory pancreatic ducts draining separately in the duodenum. This malformation is encountered in 10% of specimens. It is also an incidental finding in 3% of patients undergoing endoscopic retrograde cholangiopancreatography (ERCP).
In the subset of patients with pancreatitis, the incidence increases to 12%, suggesting that pancreas divisum predisposes to development of pancreatitis. Pancreatitis may occur due to stenosis or infarction of one or both pancreatic ducts. Others suggest that there is no association between pancreas divisum and pancreatitis. Insufficiency of the minor papilla may also play a role in development of pancreatitis.
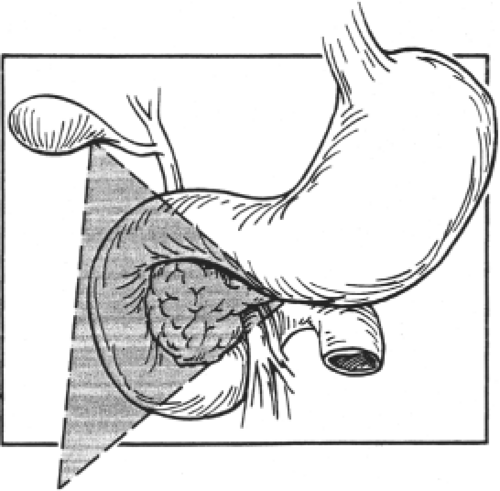
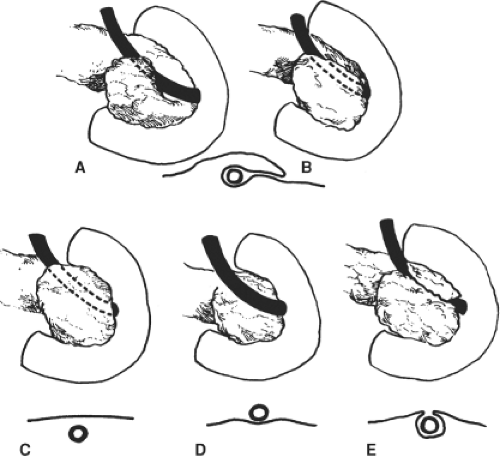
Annular pancreas: This malformation is characterized by a ring-like, thin, flat band of pancreatic tissue surrounding the second part of the duodenum at the origin of the ventral pancreas. The annulus is usually complete (Fig. 7) and may constrict the duodenum, resulting in poor gastric emptying, abdominal pain, and weight loss. The pattern of the pancreatic duct may also be affected, as there is usually a wide duct from the ring that meets the main pancreatic duct.
Several unconvincing theories have been proposed for the embryogenesis of the annular pancreas (Fig. 8). According to the theory most usually cited, an extension of the ventral pancreas may hook around the right side of the duodenum and fuse with the dorsal pancreas.
The anomaly is congenital; the affected neonates present with bilious vomiting. The radiographic finding of a “double bubble” (air in the stomach and upper duodenum) with no air caudally signifies complete obstruction of the duodenum. The condition can also be encountered in adults, as 50% of
patients present with symptoms in adulthood. Duodenoduodenostomy, or duodenojejunostomy is the proper surgical correction. Division of the annular ring will likely result in injury to the pancreatic duct and should not be performed.
patients present with symptoms in adulthood. Duodenoduodenostomy, or duodenojejunostomy is the proper surgical correction. Division of the annular ring will likely result in injury to the pancreatic duct and should not be performed.
Ectopic, heterotopic, and accessory pancreas: Ectopia and heterotopias of the pancreas signify development of pancreatic tissue in abnormal position. The term accessory pancreas is used when the original pancreas is in its normal place and there is additional pancreatic tissue in other positions. Accessory pancreatic tissue may be found in the neighboring organs such as the stomach and duodenal wall. Less frequently, the umbilicus, ileum, colon, gallbladder, or omentum are affected. Accessory pancreatic tissue can be found in Meckel’s diverticulum, which is a congenital, true diverticulum, found on the antimesenteric border of the bowel, 2 feet from the ileocecal valve. Heterotopic pancreatic tissue can also be found on Meckel’s diverticulum.
In most cases the accessory pancreatic tissue is functional. Several theories regarding development of accessory pancreas have been described, including metaplasia of pluripotent endodermal cells into pancreatic cells and transplantation of pancreatic cells into the duodenum.
Neuroendocrine tumors of the pancreas: It is unclear if these tumors are congenital; however, a discussion regarding their localization facilitates understanding of pancreatic endocrine development. These tumors develop in two distinct regions of the pancreas that, topographically, correspond to portions of the ventral and the dorsal anlagen.
Topographically, the area originating from the ventral pancreatic diverticulum is known as the gastrinoma triangle (Fig. 9), which is bound by the cystic duct, the border of the second and third part of the duodenum, and the junction of the neck and body of the pancreas. In this triangle, 80% of gastrinomas, pancreatic polypeptidomas, and somatostatinomas are encountered, while insulinomas and glucagonomas, which develop from the islets concentrated in the dorsal bud, are more common (80%) in the neck, body, and tail.
Localization of gastrinomas in peripancreatic sites, in the duodenum, and at the head of the pancreas is compatible with an origin from the ventral pancreas. It has been suggested that stem cells dispersed during the turn of the pancreatic diverticulum, and were entrapped by lymphoid tissue or the duodenal wall. This idea explains the increased localization of gastrinomas in sites outside the pancreas (40%) and in the lymph nodes (40%). These gastrinomas are distinct, as they are more often associated with the development of hepatic metastases and poorer prognosis.
Parts of the Pancreas
Anatomical Parts
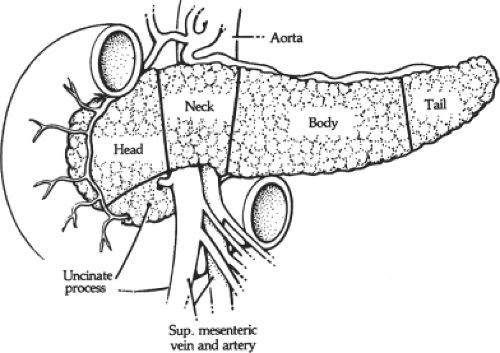
Head
The head of the pancreas is flat and has an anterior and a posterior surface (Fig. 10). The anterior surface is adjacent to the pylorus
and the transverse colon. The anterior pancreaticoduodenal arcade can be seen on the ventral surface of the head of the pancreas, coursing roughly parallel to the duodenal curvature.
and the transverse colon. The anterior pancreaticoduodenal arcade can be seen on the ventral surface of the head of the pancreas, coursing roughly parallel to the duodenal curvature.
The posterior pancreaticoduodenal vascular arcade runs along the posterior surface of the head. This surface of the pancreatic head is close to the hilum and medial border of the right kidney, the right renal vessels and the inferior vena cava, the right crus of the diaphragm, and the right gonadal vein.
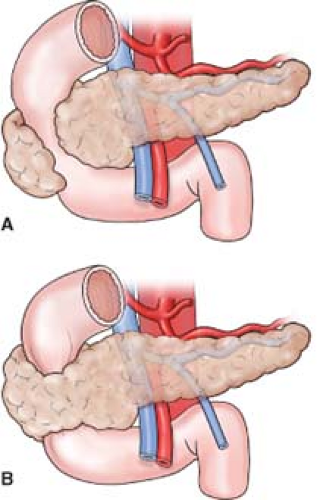
The head of the pancreas may be related to the third part of the common bile duct (CBD) in a variety of ways (Fig. 11).
Uncinate Process
An extension of the head of the pancreas (which is variable in size and shape) passes downward and slightly to the left from the principal part of the head. It further continues behind the superior mesenteric vessels and in front of the aorta and inferior vena cava. In sagittal section, the uncinate process lies between the aorta and the superior mesenteric artery (SMA), with the left renal vein above and the duodenum below (Fig. 12). If the junction of the superior mesenteric and portal vein is low, the anterior surface of the uncinate process is related to the superior mesenteric vessels and the portal vein.
The uncinate process may be absent, or it may completely encircle the superior mesenteric vessels (Fig. 13). If the process is well developed, the neck of the pancreas must be sectioned from the front to avoid injury to the vessels. Short vessels from the SMA and superior mesenteric vein (SMV) supply the uncinate process, and must be carefully ligated and divided during a pancreatic head resection. An anomalous right hepatic artery (accessory or replaced) may pass through the uncinate process.
Neck
The neck of the pancreas is defined by the location of the superior mesenteric vessels running behind the gland, and by the beginning of the portal vein dorsal to the pancreas. This pancreatic segment is 1.5 to 2.0 cm long. It is partially covered anteriorly by the pylorus. The gastroduodenal artery passes to the right of the neck and provides origin for the anterior superior pancreaticoduodenal artery. Posterior to the neck, the portal vein is formed by the confluence of the superior mesenteric and splenic veins. Near the inferior margin of the pancreatic neck, one can often see the terminations of the inferior pancreaticoduodenal vein and right gastroepiploic vein where they drain into the superior mesenteric or splenic veins or into the portal vein proper. The inferior mesenteric vein drains, with essentially equal frequency, into the splenic vein, the SMV, or the portal vein. Elevation of the neck and ligation of any anterior tributaries of the portal vein, if present, are necessary for many pancreatic resections.
The portal vein receives the posterior superior pancreaticoduodenal, right gastric, left gastric, and pyloric veins. It is fairly common for an anomalous vein to enter the anterior surface.
Body
The anterior surface of the body of the pancreas is covered by the two layers of peritoneum of the omental bursa that separates the stomach from the pancreas. The omental tuberosity (tuber omentale), a blunt upward projection from the body, contacts the lesser curvature of the stomach at the attachment of the lesser omentum. The body is also related to the transverse mesocolon, which is double-layered; the superior leaf covers the anterior surface and the inferior leaf passes inferior to the pancreas. The middle colic artery emerges from beneath the pancreas to travel between the leaves of the mesocolon.
Posteriorly, the body is related to the aorta, the origin of the SMA, the left crus of the diaphragm, the left kidney and its vessels,
the left adrenal gland, and the splenic vein. Small vessels from the pancreas enter this vein. They must be ligated during pancreatectomy if the splenic vein and the spleen are to be preserved.
the left adrenal gland, and the splenic vein. Small vessels from the pancreas enter this vein. They must be ligated during pancreatectomy if the splenic vein and the spleen are to be preserved.
Tail
The tail of the pancreas is relatively mobile; its tip reaches the hilum of the spleen in 50% of cases, lies above the hilum in 8%, and below in 42%. Together with the splenic artery and the origin of the splenic vein, the tail is contained between two layers of the splenorenal ligament. The anterior layer of this ligament is close to the posterior layer of the gastrosplenic ligament, which contains the short gastric vessels. Commonly, a caudate branch arises from the left gastroepiploic or an inferior splenic polar branch and passes to the tip of the tail of the pancreas passing from the gastrosplenic into the spernorenal ligament. The vascular anatomy in this location is quite variable. Care should be taken during dissection to avoid inadvertent vascular injury, especially during a laparoscopic approach.
Segments
Two segments have been suggested, similar to those of the liver. The right (cephalocervical)
segment is supplied by the arcades of gastrodudenal and superior mesenteric arteries. The left (corporocaudate) segment is supplied by the splenic and dorsal pancreatic artery. The two segments are separated by a poorly vascularized area. They are connected by the pancreatic duct and often by a small artery connecting the dorsal pancreatic artery with the posterior arcade (see Fig 19). Both segments can be used for transplantation.
segment is supplied by the arcades of gastrodudenal and superior mesenteric arteries. The left (corporocaudate) segment is supplied by the splenic and dorsal pancreatic artery. The two segments are separated by a poorly vascularized area. They are connected by the pancreatic duct and often by a small artery connecting the dorsal pancreatic artery with the posterior arcade (see Fig 19). Both segments can be used for transplantation.
Surgical Considerations
Sixty to seventy percent of the gland resides to the left of the portal vein–SMV junction; thus, a typical Whipple resection results in approximately a 35% gland removal.
Even with an 80% pancreatectomy, good exocrine and endocrine activity are possible; however, a parenchyma-sparing technique is preferred when possible (Fig. 14).
Pancreatic Ducts
Anatomy and Variations
The pancreatic ducts lie anterior to the major pancreatic vessels. The main pancreatic duct arises in the tail of the pancreas. Through the tail and body of the pancreas, the duct lies midway between the superior and inferior margins, slightly more posterior. It crosses the vertebral column between the 12th thoracic and 2nd lumbar vertebrae.
Fifteen to twenty short tributaries enter the duct at right angles in the region of the body and tail; superior and inferior tributaries tend to alternate. The main duct may receive a tributary draining the uncinate process, and, in some individuals, the accessory pancreatic duct empties into the main duct. Small tributary ducts in the head may open directly into the intrapancreatic portion of the CBD.
The main duct turns caudad and posterior upon reaching the head or neck of the pancreas. At the level of the major papilla, the duct turns horizontally to join the caudal surface of the CBD and enters the wall of the duodenum, usually at the level of the second lumbar vertebra.
The accessory pancreatic duct (of Santorini) may drain the anterosuperior portion of the head, either into the duodenum at the minor papilla or into the main pancreatic duct. Because of the developmental origin of the two pancreatic ducts, several variations are encountered. The usual configuration is as seen in Figure 15A. The accessory duct (of Santorini) is smaller than the main pancreatic duct (of Wirsung) and opens into the duodenum on the minor papilla. Configurations in Figure 15B to E show examples of progressive reduction in the size of the accessory duct.
In approximately 10% of individuals, there is no connection between the accessory duct and the main duct (Fig. 15D). This fact is important to remember when contrast medium is injected into the main duct. There is no minor papilla in 30% of individuals (Fig. 15B,C,E). In some individuals who have a minor papilla, the terminal portion of the accessory duct is too small to permit the passage of any quantity of fluid. Three papillae have been seen.
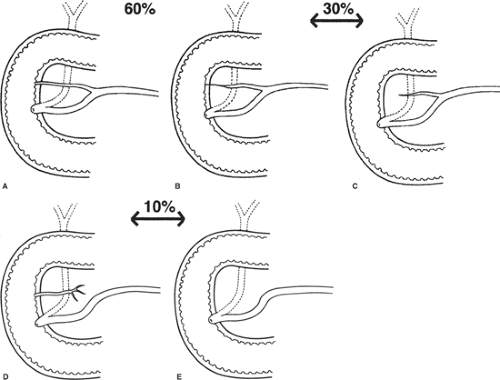 Fig. 15. Variations of the pancreatic ducts. Degrees of suppression of the accessory duct. A: Both ducts open into the duodenum (60%). B,C: The accessory duct ends blindly (30%). D: The accessory duct does not communicate with the main duct (10%). E: The accessory duct is absent. (From Skandalakis JE, Gray SW, Rowe JS Jr., et al. Anatomical complications of pancreatic surgery. Contemp Surg 1979;15:17.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|