Janice A. Neil
Surgery of the Liver, Biliary Tract, Pancreas, and Spleen
A pathologic condition in the liver, biliary tract, pancreas, or spleen often requires surgical intervention. These organs are highly vascular and control many of the body’s metabolic and immune functions. Surgical intervention may be indicated for infection, cystic anomalies, congenital anomalies, metabolic diseases, trauma (see Chapter 28), or malignancy. Many new cases of malignancy of the gallbladder, pancreas, or extrahepatic biliary tract are diagnosed each year and the prognosis for these is often poor (Jackson and Evans, 2012; Jensen et al, 2012). Pancreatic cancer remains the fourth leading cause of death in the United States (National Cancer Institute, 2011). Surgeries of the liver and biliary tract have become more advanced as research and new technology permit more complete diagnoses of pathologic conditions. Resection of the liver for carcinoma has achieved a recognized role for cure or substantial palliation with safety and low morbidity.
Cholecystectomy is the most common, nonemergency abdominal operation performed in the United States (Ingraham et al, 2011). Laparoscopic cholecystectomy is now the gold standard surgical intervention for the treatment of cholecystitis. Compared with open-incision cholecystectomy, laparoscopic cholecystectomy results in reduced trauma to tissues as well as shorter postoperative recoveries, both distinct advantages. About 94% of cholecystectomies are elective surgeries; the remaining 6% are emergencies (Overby et al, 2010). Laparoscopic cholecystectomies were the precursor to numerous abdominal procedures now performed or assisted with the laparoscope.
New diagnostic technology and intraoperative use of ultrasonography, biliary endoscopy, and radiography enable surgeons to better treat diseases of the biliary tract. Solid organ transplantation, such as for the liver and pancreas, is a common means to treat primary hepatic tumors, end-stage liver disease, and insulin-deficient diabetes. Liver transplant procedures include entire organ transplants as well as living-related organ donations.
This chapter explores the most common open and minimally invasive procedures performed on the liver, biliary tract, pancreas, and spleen.
Surgical Anatomy
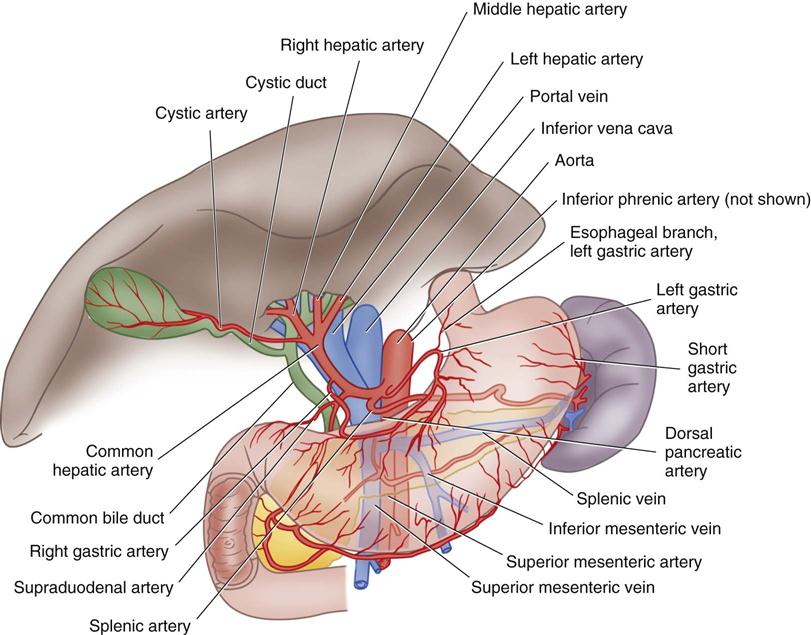
The liver is in the right upper quadrant of the abdominal cavity, beneath the dome of the diaphragm and directly above the stomach, duodenum, and hepatic flexure of the colon. The external covering, known as Glisson’s capsule, is composed of dense connective tissue. The visceral peritoneum extends over the entire surface of the liver, except at its posterior attachment to the diaphragm. This connective tissue branches at the porta hepatis into a network of septa that extends into an intrahepatic network of support for the more than 1 million hepatic lobules. The porta hepatis is located on the inferior surface of the liver and provides entry and exit for the major vessels, ducts, and nerves. The hepatic artery maintains the arterial blood supply. Venous blood from the stomach, intestines, spleen, and pancreas travels to the liver by the portal vein and its branches (Figure 12-1), and the hepatic venous system then returns blood to the heart via the inferior vena cava.
Lobules are the functional units of the liver. Each lobule contains a portal triad that consists of a hepatic duct, a hepatic portal vein branch, and a branch of the hepatic artery, nerves, and lymphatics. A central vein is located in the center of each lobule and provides venous drainage into the hepatic veins.
Lobules also contain hepatic cords, hepatic sinusoids, and bile canaliculi. The hepatic cords consist of numerous columns of hepatocytes—the functional cells of the liver. The hepatic sinusoids are the blood channels that communicate among the columns of hepatocytes. The sinusoids have a thin epithelial lining composed primarily of Kupffer cells—phagocytic cells that engulf bacteria and toxins. The sinusoids drain into the central vein.
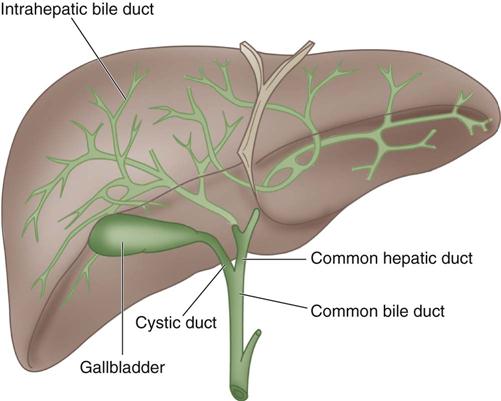
Bile is manufactured by the hepatocytes. The bile canaliculi are tiny bile capillary vessels that communicate among the columns of hepatocytes. The bile canaliculi collect and transport bile to the bile ducts in the portal triad of each lobule, from which bile then flows into the hepatic ducts at the porta hepatis. These ducts join immediately to form one common hepatic duct that merges with the cystic duct from the gallbladder to form the common bile duct (Figure 12-2). The common bile duct opens into the duodenum in an area called the ampulla, or papilla of Vater, located about 7.5 cm below the pyloric opening from the stomach.
Bile contains bile salts, which facilitate digestion and absorption, and various waste products. The liver is essential in the metabolism of carbohydrates, proteins, and fats. It metabolizes nutrients into stores of glycogen, used for regulation of blood glucose levels and as energy sources for the brain and body functions.
The liver plays several important roles in the blood-clotting mechanism. It is the organ that synthesizes plasma proteins, excluding gamma globulins but including prothrombin and fibrinogen. Vitamin K, a cofactor to the synthesis of prothrombin, is absorbed by the metabolism of fats in the intestinal tract as a result of bile formation by the liver. Patients with liver disease may have altered blood-coagulation abilities.
The liver also synthesizes lipoproteins and cholesterol. Cholesterol is an essential component of the blood plasma. It serves as a precursor for bile salts, steroid hormones, plasma membranes, and other specialized molecules. A diet high in cholesterol reduces the amount that must be synthesized by the liver. When the diet is deficient in cholesterol, the liver increases synthesis to maintain levels necessary for production of vital chemical molecules.
The liver also functions in the metabolic alteration of foreign molecules or biotransformation of chemicals. The microsomal enzyme system (MES) plays a major role in the body’s response to foreign chemicals, such as pollutants, drugs, and alcohol. Patients with liver disease may have an altered response to chemical substances. This consideration is important in the induction and management of general anesthesia for patients with liver disorders.
The gallbladder, which lies in a sulcus on the undersurface of the right lobe of the liver, terminates in the cystic duct (Figure 12-3). This ductal system provides a channel for the flow of bile to the gallbladder, where it becomes highly concentrated during storage. The liver produces about 600 to 1000 mL of bile each day. The gallbladder’s average storage capacity is 40 to 70 mL. As foods, especially fats, are ingested, the duodenal cells release cholecystokinin. When the musculature of the gallbladder contracts, bile is forced into the cystic duct and through the common duct. As the sphincter of Oddi in the ampulla of Vater relaxes, bile is released, flowing into the duodenum to aid in digestion by emulsification of fats. The gallbladder receives its blood supply from the cystic artery, a branch of the hepatic artery. The triangle of Calot contains the cystic artery (and sometimes the right hepatic artery); it is an anatomic landmark in surgical removal of the gallbladder (Jackson and Evans, 2012). Its boundaries may be remembered as the “3 Cs”: cystic duct, common hepatic duct, and cystic artery. Innervation for the gallbladder and biliary tree is controlled by the autonomic nervous system. Parasympathetic innervation stimulates contraction, whereas sympathetic innervation inhibits contraction.
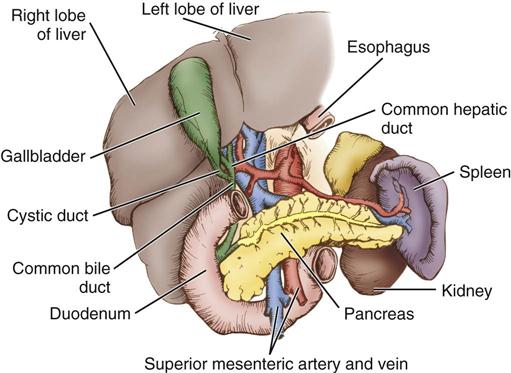
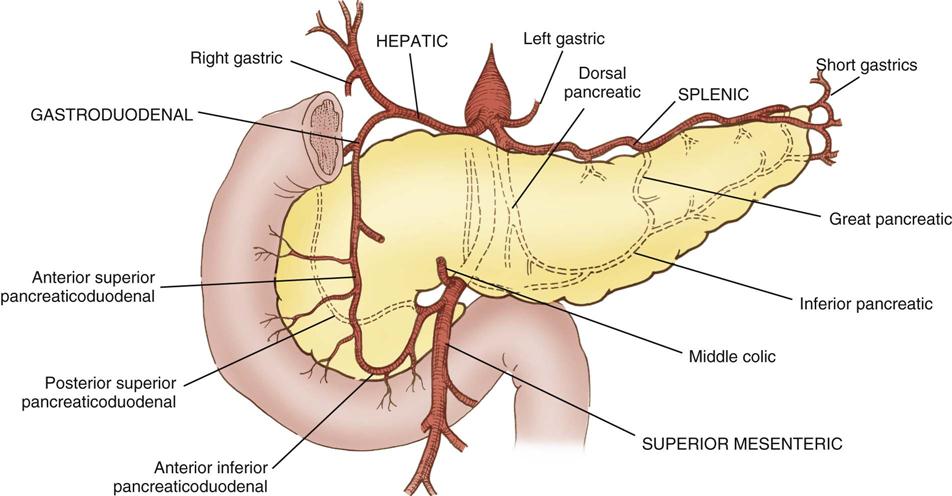
The pancreas (see Figure 12-3) is a fixed structure lying transversely behind the stomach in the upper abdomen. The head of the pancreas is fixed to the curve of the duodenum. Blood is supplied to the pancreas and the duodenum from the celiac axis and superior mesenteric artery (Figure 12-4). The body of the pancreas lies across the vertebrae and over the superior mesenteric artery and vein. The tail of the pancreas extends to the hilum of the spleen. In total, the pancreas extends about 25 cm. Pancreatic secretions containing digestive enzymes are collected in the pancreatic duct, or duct of Wirsung, which joins with the common bile duct to enter the duodenum about 7.5 cm below the pylorus. The dilated junction of the two ducts at the point of entry forms the ampulla of Vater.
The pancreas also contains groups of cells, called islets, or islands, of Langerhans, that secrete hormones into the blood capillaries instead of into the duct. These hormones are insulin and glucagon, and both are involved in carbohydrate metabolism.
The spleen (Figure 12-5) is in the upper left abdominal cavity, with full protection provided by the tenth, eleventh, and twelfth ribs; its lateral surface is directly beneath the dome of the diaphragm. The anterior medial surface is in proximity to the cardiac end of the stomach and the splenic flexure of the colon. The spleen is covered with peritoneum that forms supporting ligaments. The splenic artery, a branch of the celiac axis, furnishes the arterial blood supply. The splenic vein drains into the portal system.
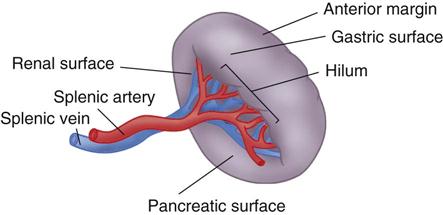
The spleen has many functions. Among them are defense of the body by phagocytosis of microorganisms, formation of nongranular leukocytes and plasma cells, and phagocytosis of damaged red blood cells. It also acts as a blood reservoir.
Perioperative Nursing Considerations
Assessment
The patient with hepatobiliary disease may have extreme jaundice, urticaria, petechiae, lethargy, and irritability. Depending on the extent of the disease, bleeding and coagulation times may increase and the platelet count decrease, contributing to intraoperative concerns with achieving hemostasis. A thorough nursing history is necessary for proper assessment of the health status of patients with dysfunctions of the hepatobiliary system, pancreas, or spleen. Assessment includes data about the patient’s history of chronic disease, current medications, perceptions of his or her disease, comfort status, nutritional status, fluid and electrolyte balance, bowel and elimination patterns, energy level and independence, and exposure to toxins. Many industrial compounds are toxic to the liver (Table 12-1).
TABLE 12-1
Common Hepatotoxic Agents and Types of Liver Damage
| Toxic Agent | Source | Liver Damage |
| Aflatoxin B | Moldy foods, rice, corn, cassava, oil, grain dust (during bin clean-out and animal feeding in enclosed buildings) | Jaundice, fatty liver, hepatocellular carcinoma, thromboses |
| Amanita phalloides | Poisonous mushrooms | Centrilobular and massive necrosis |
| Benzene | Chemical industry, to make plastics, resins, and nylon and synthetic fibers | Fatty liver, cirrhosis |
| Beryllium | From x-ray tube and fluorescent lamp manufacture; alloys are used in automobiles, computers, sports equipment (golf clubs and bicycle frames) | Necrosis and granulomas |
| Boron, cadmium, nickel, chromium, copper | From gold smelting, plating | Liver damage, rise in levels of liver enzymes |
| Carbon tetrachloride | Propellants for aerosol cans, as a pesticide, cleaning fluid, degreasing agent, fire extinguishers, spot removers (now banned) | Centrilobular necrosis |
| Kerosene | From fuel handling | Liver damage, rise in levels of liver enzymes |
| Lead | Environment | Steatosis, hepatitis |
| Pesticides | Polyvinyl chloride, farming industry | Steatosis, angiosarcoma (liver tumors) |
| Phosphorus | From poisons and firecrackers | Fatty liver, necrosis, fibrosis |
| Toluene, xylene | Occurs naturally in crude oil | Fatty liver, fibrosis |
| Vinyl chloride | Used to make polyvinyl chloride (plastics; also named PVC) | Angiosarcoma (liver tumors), fibrosis |
Modified from Orfei L: Toxic liver injury, 2012, available at www.meddean.luc.edu/lumen/MedEd/orfpath/toxicinjury.htm. Accessed January 28, 2013; Aflatoxin B1 exposure, 2009, available at www.ncbi.nlm.nih.gov/pubmed/19273485. Accessed January 28, 2013; Amanita phalloides, 2012, available at www.ncbi.nlm.nih.gov/pubmed/22811920, accessed January 28, 2013; Toxic Substances Portal, available at www.atsdr.cdc.gov/toxicsubstances.html. Accessed January 28, 2013.
Establishing an objective database for a person with hepatobiliary or pancreatic dysfunction requires particular attention to characteristic signs of organ dysfunction (Evidence for Practice). Increased abdominal girth and distention, palmar erythema, distended periumbilical veins, hemorrhagic areas, spider nevi, muscle wasting, and dry mucous membranes are some characteristic signs and symptoms. Vascular volume is assessed by noting vital signs, including any orthostatic changes, skin turgor, temperature, and appearance, as well as weight gain or loss. Physical examination of the patient’s abdomen includes palpation and percussion to evaluate tenderness, ascites, and organ enlargement.
Common laboratory tests to assess liver function are those that evaluate fat metabolism, protein metabolism, blood coagulation properties, bilirubin metabolism, and antigens and antibodies of hepatitis (Appendix A). Common tests of pancreatic function also can be found in Appendix A. Radiographic studies commonly used to evaluate function of the liver, pancreas, and spleen include abdominal examination, upper gastrointestinal (GI) series, ultrasound studies, computed tomography (CT) scan, radioisotope scanning, nuclear magnetic resonance (NMR) imaging, angiography, cholecystography, and cholangiography. The hepatic iminodiacetic acid (HIDA) scan can be used to evaluate the physiologic secretion of bile. State-of-the-art imaging includes contrast-enhanced MRI, MR cholangiopancreatography (MRCP), endoscopic ultrasound (EUS), and high-resolution, thin-section spiral CT for imaging pancreatic and biliary structures. In addition, fluorodeoxyglucose positron emission tomography (FDG-PET) is a whole-body technique that can detect metastases, recognition of which may change surgical management (Jackson and Evans, 2012).
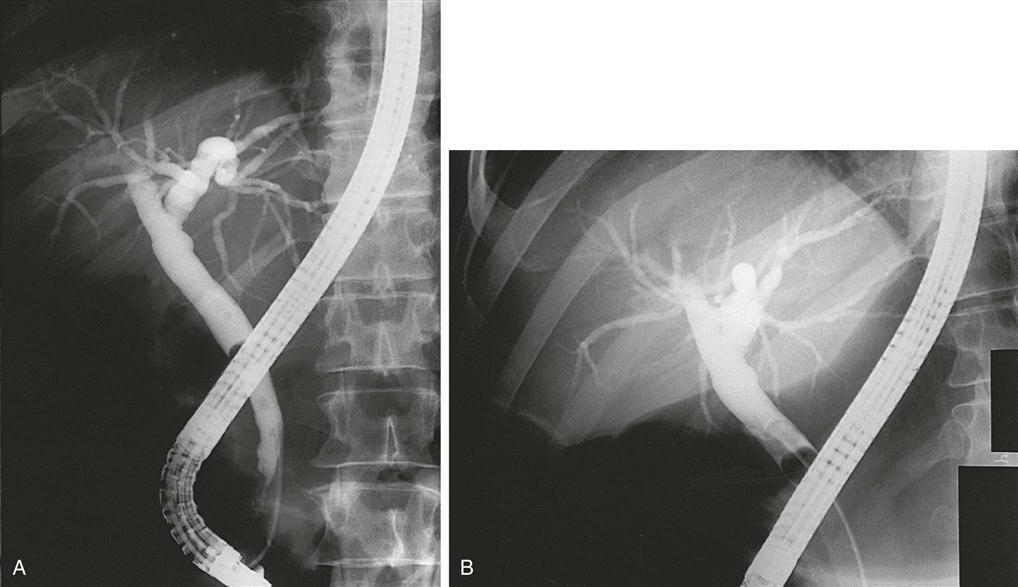
Endoscopy and biopsy are more invasive diagnostic procedures. Endoscopic retrograde cholangiopancreatography (ERCP) (Figure 12-6) allows direct visualization of the biliary tract, injection of radiographic dye into the ductal system, and biopsy when indicated. Percutaneous transhepatic cholangiography (PTC) uses percutaneous insertion of a long flexible needle into a bile duct of the liver. Contrast medium is injected and serial x-ray examination is performed. Arteriography of the liver, biliary tree, pancreas, and spleen requires femoral arteriotomy and placement of a catheter into the celiac branch of the abdominal aorta under fluoroscopic visualization. Contrast medium is then injected and serial x-ray examination allows the vessels to be visualized during the perfusion and drainage phases.
Nursing Diagnosis
After reviewing the nursing assessment, the perioperative nurse formulates nursing diagnoses. Nursing diagnoses related to the care of patients undergoing surgery of the liver, biliary tract, pancreas, or spleen might include the following:
• Anxiety related to impending surgical procedure, perioperative events, and surgical outcome
• Risk for Imbalanced Fluid Volume
• Risk for Infection related to organ systems involved (portions of the GI tract)
• Risk for Perioperative Positioning Injury
• Risk for Impaired Skin Integrity related to invasion of body structures, disruption of skin surface
Outcome Identification
Statements about desired outcomes reflect nursing diagnoses identified for a patient population. Nursing diagnoses are also individualized according to cultural, ethnic, religious, and spiritual values, as well as an individual patient’s status. From these are derived the outcomes the perioperative nurse wishes to achieve. The best outcome statement has specific criteria by which the perioperative nurse intends to measure whether the outcome has been met. These criteria are more meaningful when they are established in partnership with the patient. Not all outcomes will be planned with the patient, but ones relating to nursing diagnoses such as anxiety and coping can and should reflect patient participation. Outcomes identified for the selected nursing diagnoses might be stated as follows:
• The patient will maintain fluid volume equilibrium throughout the operative procedure.
• The patient will be free of clinical signs and symptoms of surgical site infection (SSI).
• The patient will demonstrate understanding of the plan to heal the incision site.
• The patient will report that the pain management regimen relieves pain to a satisfactory level (Ackley and Ladwig, 2011).
Planning
Planning the patient’s care requires knowledge of the anatomy and subsequent physiologic complications that may occur with surgical interruption of tissues. Planning is driven by intended outcomes. The experienced perioperative nurse reflects on those intended outcomes and, using theory, science, and what has been learned through experience, identifies actions required for outcome achievement (Benner et al, 2009). Principles of safe surgical positioning, maintenance of asepsis, prevention of biologic and electrical hazards, and provision of proper instrumentation and equipment are a few constituents of the plan of care that are based on theory and science and augmented by nursing experience.
A review of the nursing assessment and a patient interview provide insights as to the specific needs of the patient. The patient’s medical and surgical history, as well as age, size, and nutritional status, will assist the perioperative nurse in developing an effective plan of care. A Sample Plan of Care for a patient undergoing surgery of the liver, biliary tract, pancreas, or spleen is shown below.
Implementation
Patients having surgery of the liver, biliary tract, pancreas, or spleen are usually given a general anesthetic. The following pertinent factors should be considered in caring for these patients.
Universal Protocol.
The Joint Commission (TJC) requires that the “wrong site, wrong procedure, wrong person” prevention protocol be carried out before each surgical procedure (TJC, 2012). This protocol is discussed in Chapter 2.
Positioning the Patient.
For biliary surgery the patient is placed in supine position. Arms are placed on padded armboards with the palms up and fingers extended. Armboards are maintained at less than a 90-degree angle to prevent brachial plexus stretch. If there are surgical reasons to tuck the arms at the side, the elbows are padded to protect the ulnar nerve, the palms faced inward, and the wrists maintained in a neutral position (Phillips, 2013). A drape secures the arms. It should be tucked snuggly, but not tightly, under the patient, not under the mattress. This prevents the arm from shifting downward intraoperatively and resting against the operating room (OR) bed rail. A small positioning aid may be placed under the lower right side of the thorax to elevate the lower rib cage, providing better exposure and access to the viscera in the right upper quadrant of the abdomen. Alternatively, a lateral tilt of the OR bed may be used in combination with reverse Trendelenburg for procedures such as laparoscopic cholecystectomy.
Positioning for laparoscopic procedures requires the perioperative nurse to exercise caution when applying safety straps. Given that the patient may be placed in a severe side tilt or reverse Trendelenburg position, safety or restraining straps must be placed securely, but not too tightly. Attention is given to proper alignment of the patient’s body and extremities, and padded footboards are applied to prevent the patient from slipping. Areas of pressure in the selected surgical position (see Chapter 6) and bony prominences are padded well to prevent interruption of circulation and pressure injury to tissues and neurovascular structures. These precautions are especially important for diabetic, circulatory-impaired, immunocompromised, and elderly patients. Close monitoring of the patient is essential during positional changes, especially in laparoscopic procedures with decreased lighting in the room.
When anticipating an operative cholangiogram, the perioperative nurse must ensure that the OR bed has been equipped and positioned so that C-arm image intensification can be accomplished efficiently. Radiation-protection devices for the surgical team and patient should be available and applied/worn as indicated.
Thermoregulation.
The risks of intraoperative hypothermia have been well documented (see Chapter 5). When laparotomy is performed, patients are at further risk for hypothermia. To prevent unplanned hypothermia, the perioperative nurse takes affirmative measures to maintain body temperature in the OR. Proper environmental temperature and humidity prevent body heat loss caused by evaporation and convection. A forced-air warming blanket placed over the patient’s upper body, head, and neck assists in maintaining body temperature. Minimizing body exposure to ambient air and using warm irrigating solutions also support thermoregulation. The temperature of irrigating fluids should be no higher than body temperature (98.6° F [37° C]) (AORN, 2013). A blood- and fluid-warming device may be used by the anesthesia provider to deliver intravenous (IV) fluids at a temperature higher than room air temperature (see Chapter 5). The anesthesia provider commonly monitors the patient’s core temperature by use of an esophageal temperature probe when duration and complexity of the surgical procedure place the patient at risk for hypothermia. Additional comfort measures include using warm blankets before and after surgery.
Application of Intermittent Pneumatic Compression Device.
Patients undergoing lengthy surgical procedures are at risk for venous dilation and blood pooling in the lower extremities. This may predispose the surgical patient to develop venous thromboembolism (VTE) in the postoperative period. Intermittent pneumatic compression devices (IPCDs) in conjunction with graduated compression stockings (referred to as mechanical prophylaxis) are applied in the OR before commencing lengthy surgical procedures in order to prevent or minimize VTE risks (Alexander-Magalee, 2013).
Draping the Patient.
After the abdominal prep the surgical team must allow time for the prep solution to dry and vapors to dissipate. This is an essential patient safety precaution when flammable prep solutions are used in conjunction with electrosurgery (or other ignition sources, such as a laser). Sterile towels are then arranged to accommodate the intended incision. A sterile drape sheet may be placed over the patient’s lower torso and a laparotomy sheet then placed to provide a wide sterile field and to cover all exposed body surfaces except the incision site. Further information on draping can be found in Chapter 4.
Instrumentation.
Instrumentation for open (i.e., performed via a laparotomy incision) surgeries of the liver, biliary tract, spleen, and pancreas includes a basic laparotomy set, biliary probes and forceps for dilating and exploring the ducts of the pancreas and biliary tract, vascular clamps, GI clamps, and ligating clips and appliers of all sizes. Linear stapling instruments also should be available. A self-retaining system such as the Bookwalter retractor set (Figure 12-7) provides excellent exposure of the abdominal viscera. In addition, a flexible choledochoscope, Cavitron ultrasound suction aspirator (CUSA), intraoperative ultrasound, laser, argon beam coagulator, harmonic scalpel, and electrosurgical unit (ESU) may be required to perform certain procedures on the hepatobiliary system. Safe practices when using devices that generate surgical smoke require the use of a smoke evacuation system and accessories in both open and laparoscopic procedures (AORN, 2013).
The basic equipment for minimally invasive surgery (MIS) procedures consists of two high-density monitors, an insufflation unit, ESU, light source, camera, and 0- and 30-degree telescopes in 10- and 5-mm sizes. A printer is optional. An ultrasonic dissecting unit is often used with MIS procedures. Trocars and sleeves are available in reusable, disposable, and reposable designs. Trocars and sleeves are commonly designed to accommodate 10- to 5-mm instruments and 12- to 5-mm accessories and instruments. MIS instruments include scissors and shears, dissecting forceps, atraumatic grasping forceps, hooks, Babcock clamps, retractors, needles, suturing devices, pouches, suction-irrigating devices, and mechanical stapling devices.
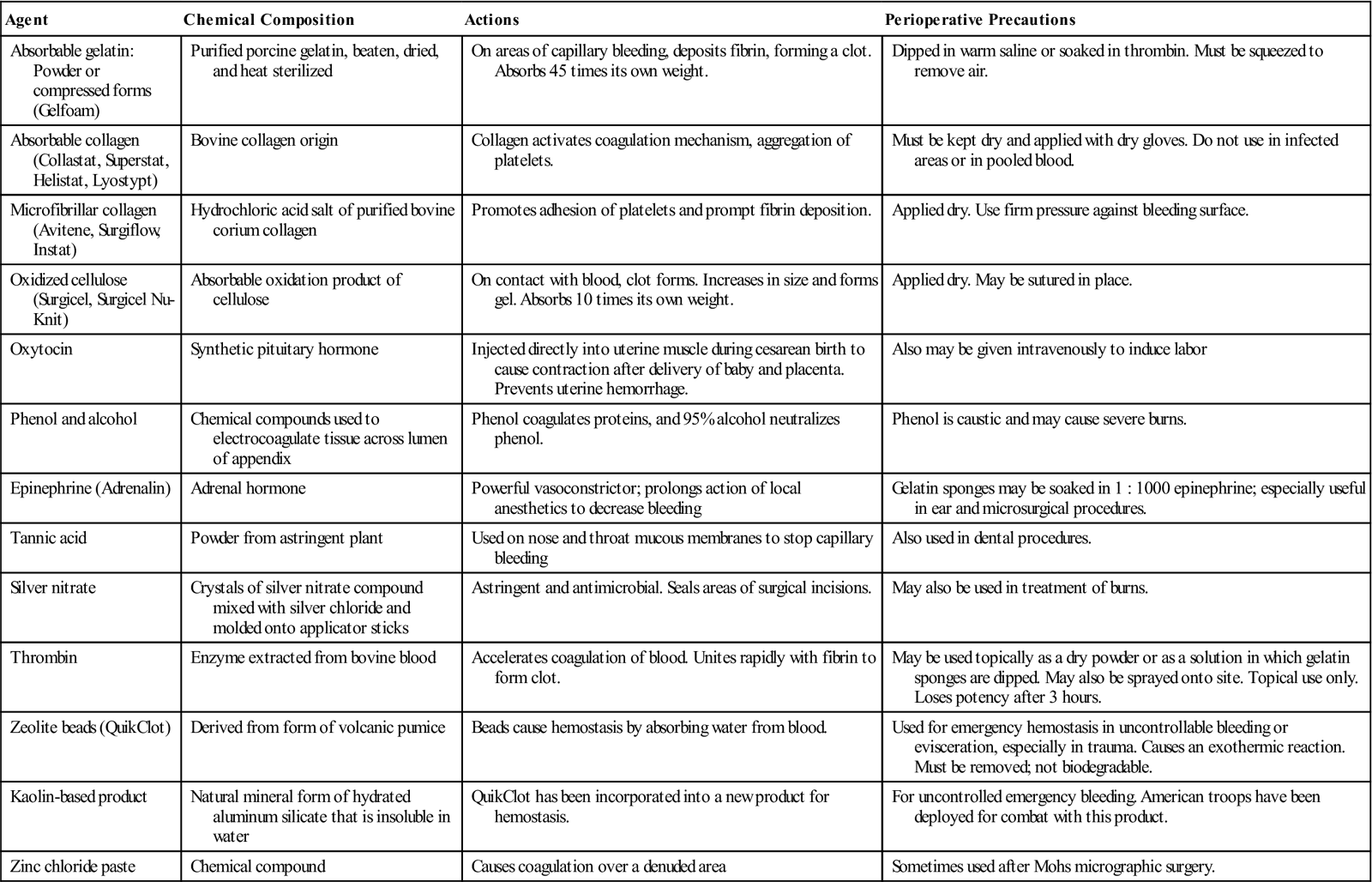
Thrombin, Gelfoam, Surgicel, Avitene, and other chemical hemostatic agents (Surgical Pharmacology) should be available in the OR suite. Radiographic dye, supplies, and radiation-protection devices are required if intraoperative radiography or angiography is planned as part of the procedure.
Surgical Pharmacology
Chemical Hemostatic Agents
| Agent | Chemical Composition | Actions | Perioperative Precautions |
| Absorbable gelatin: Powder or compressed forms (Gelfoam) | Purified porcine gelatin, beaten, dried, and heat sterilized | On areas of capillary bleeding, deposits fibrin, forming a clot. Absorbs 45 times its own weight. | Dipped in warm saline or soaked in thrombin. Must be squeezed to remove air. |
| Absorbable collagen (Collastat, Superstat, Helistat, Lyostypt) | Bovine collagen origin | Collagen activates coagulation mechanism, aggregation of platelets. | Must be kept dry and applied with dry gloves. Do not use in infected areas or in pooled blood. |
| Microfibrillar collagen (Avitene, Surgiflow, Instat) | Hydrochloric acid salt of purified bovine corium collagen | Promotes adhesion of platelets and prompt fibrin deposition. | Applied dry. Use firm pressure against bleeding surface. |
| Oxidized cellulose (Surgicel, Surgicel Nu-Knit) | Absorbable oxidation product of cellulose | On contact with blood, clot forms. Increases in size and forms gel. Absorbs 10 times its own weight. | Applied dry. May be sutured in place. |
| Oxytocin | Synthetic pituitary hormone | Injected directly into uterine muscle during cesarean birth to cause contraction after delivery of baby and placenta. Prevents uterine hemorrhage. | Also may be given intravenously to induce labor |
| Phenol and alcohol | Chemical compounds used to electrocoagulate tissue across lumen of appendix | Phenol coagulates proteins, and 95% alcohol neutralizes phenol. | Phenol is caustic and may cause severe burns. |
| Epinephrine (Adrenalin) | Adrenal hormone | Powerful vasoconstrictor; prolongs action of local anesthetics to decrease bleeding | Gelatin sponges may be soaked in 1 : 1000 epinephrine; especially useful in ear and microsurgical procedures. |
| Tannic acid | Powder from astringent plant | Used on nose and throat mucous membranes to stop capillary bleeding | Also used in dental procedures. |
| Silver nitrate | Crystals of silver nitrate compound mixed with silver chloride and molded onto applicator sticks | Astringent and antimicrobial. Seals areas of surgical incisions. | May also be used in treatment of burns. |
| Thrombin | Enzyme extracted from bovine blood | Accelerates coagulation of blood. Unites rapidly with fibrin to form clot. | May be used topically as a dry powder or as a solution in which gelatin sponges are dipped. May also be sprayed onto site. Topical use only. Loses potency after 3 hours. |
| Zeolite beads (QuikClot) | Derived from form of volcanic pumice | Beads cause hemostasis by absorbing water from blood. | Used for emergency hemostasis in uncontrollable bleeding or evisceration, especially in trauma. Causes an exothermic reaction. Must be removed; not biodegradable. |
| Kaolin-based product | Natural mineral form of hydrated aluminum silicate that is insoluble in water | QuikClot has been incorporated into a new product for hemostasis. | For uncontrolled emergency bleeding. American troops have been deployed for combat with this product. |
| Zinc chloride paste | Chemical compound | Causes coagulation over a denuded area | Sometimes used after Mohs micrographic surgery. |
Modified from Phillips NF: Berry and Kohn’s operating room technique, ed 12, St Louis, 2013, Mosby.
Drainage Materials.
Tubes and catheters are selected for the areas to be drained. If a defective drain is used, a free fragment may remain in the wound on removal of the tube. Thus the scrub person should note the condition of all drainage materials and should test them for patency before they are placed in the patient.
Soft rubber or latex tissue drains may be used after an open cholecystectomy or a choledochostomy. Verify that the patient has no latex allergy before using these devices and substitute nonlatex drains if necessary. The surgeon will prepare a latex rubber T-tube drain of suitable size after exploring the duct. The center of the crossbar is notched opposite the junction of the vertical limb so that its ends will bend more readily during removal. The ends are beveled and tailored to fit the duct.
Drains are usually exteriorized through separate stab wounds and anchored to skin edges to prevent their retraction. The perioperative nurse should document the types of drains and reservoirs inserted during the procedure. Depending on institutional protocol, these may be identified with an applied label. All drains and their locations should be included in the perioperative nurse’s hand-off report to the nursing unit to which the patient is transferred postoperatively.
Aseptic Considerations.
When the common duct is opened or an anastomosis is established between a duct and other parts of the alimentary tract, it may be the institution’s policy or the surgeon’s preference to isolate contaminated instruments and materials from the remainder of the operative field, as described for GI surgery (see Chapter 11). The wound is classified according to a standard system: any procedure in which the alimentary tract is entered under controlled conditions and without unusual contamination is considered a clean contaminated wound; if there is gross spillage, however, the wound is classified as contaminated. Proper wound classification is considered an important predictor of postoperative SSI (Phillips, 2013).
Blood Products.
During the preoperative verification process the perioperative nurse should ascertain the type and amount of blood and blood products, both requested and available, as well as ensure the patient has a signed consent for transfusion. Constant, ongoing evaluation of blood loss is communicated to the anesthesia provider and surgical team during the procedure. When additional blood or blood products are required, the perioperative nurse communicates with blood bank personnel so that products are readily available and carries out the required steps to verify blood/blood products with the anesthesia provider before transfusion.
Autologous blood or donor-directed blood products may be used in elective procedures involving the liver, pancreas, spleen, and biliary tract. Cell-saver devices may be used when potential contamination of the blood from bile or bowel does not exist.
Evaluation
Evaluation of the patient after surgery includes examination of all skin surfaces and comparison with preoperative assessment data. Abdominal drains, chest drainage systems, urinary drainage systems, and peripheral infusion lines are assessed for patency. Fluid volume use and loss are documented and communicated appropriately. A report of the patient’s history, preoperative assessment, intraoperative events, and postoperative evaluation is communicated to the postanesthesia care unit (PACU) or surgical intensive care unit (SICU) nurse during the hand-off.
Evaluation of patient status can be phrased as outcome statements such as the following:
Patient and Family Education and Discharge Planning
The length of time and complexity of recovery vary greatly for patients undergoing surgical intervention for disorders of the liver, biliary tract, pancreas, or spleen. Laparoscopic cholecystectomy may be performed on an ambulatory surgery basis with extended recovery and observation of 6 to 8 hours (Ambulatory Surgery Considerations). In contrast, patients undergoing liver transplant or resection may require extensive recovery that includes a stay in the intensive care unit.
Patients undergoing laparotomy for surgical procedures on the liver, biliary tract, pancreas, or spleen may have varying degrees of postoperative edema, decreased GI peristalsis, and alterations in tissue oxygenation and lymphatic drainage, depending on the amount of manipulation, resection, and trauma to the normal anatomic structures of these viscera. General anesthesia is commonly administered. Smooth muscle relaxation is imperative for most major abdominal procedures. The patient usually experiences decreased peristalsis for 2 to 5 days after laparotomy. A nasogastric (NG) tube or gastrostomy tube is inserted during the procedure to evacuate large volumes of gastric juices. Diet is introduced only after bowel sounds return. The patient may experience nausea and vomiting if food or oral fluid is introduced too early for the GI system to function with normal absorption and motility.
Coughing and deep breathing are important for patients recovering from general anesthesia and abdominal surgery. Splinting of the abdominal muscles and use of an incentive spirometer assists the patient in postoperative coughing and deep breathing. Early ambulation assists the patient to regain overall muscle tone and prevents VTE in the lower extremities.
Pain management is very important in the patient’s recovery and discharge planning. For most patients undergoing abdominal surgery, patient-controlled analgesia (PCA) or epidural analgesia may be used for better and more consistent control of pain and discomfort in the first 1 to 3 postoperative days. Narcotics may, however, add to the length of time for normal bowel peristalsis to return and their use is monitored closely after the third postoperative day.
General discharge instructions for the patient undergoing surgery for disorders of the liver, biliary tract, pancreas, or spleen might include the recommendations found in the Patient and Family Education box. In addition to such general instructions, surgical patients and their family or caregiver should receive surgery-specific special instructions. The discharge nurse should review medications the patient will be taking after discharge (medication reconciliation) along with purposes, dosages, schedules, and routes of administration for each, as well as any side effects to be reported. Both verbal and written instructions are provided, with phone numbers of those to call if questions arise and instructions for emergency situations. Patients should “teach back,” in their own words, all instructions and should be able to state the name of their surgical procedure. For most patients, a healthcare provider (such as the surgeon, nurse practitioner [NP] or physician assistant [PA]) should be notified if any of the following develop:
• Persistent fever (body temperature of 101° F [38.3° C] or higher)
• Bleeding
• Increased abdominal swelling or pain
• Chills
• Persistent cough or shortness of breath
• Persistent pain, redness, swelling, or purulent drainage from incision sites
Follow-up care may also require providing referrals for home care (or other) services.
Surgical Interventions
Surgery of the Biliary Tract
Laparoscopic Cholecystectomy
Cholecystectomy is removal of the gallbladder. It is performed for the treatment of diseases such as acute or chronic inflammation (cholecystitis) or stones (cholelithiasis) (Box 12-1). Most of these procedures are done laparoscopically. Laparoscopic cholecystectomy is the surgical treatment of choice for patients with gallbladder disease who meet the appropriate criteria for safe laparoscopic intervention. Preoperative evaluation of patients having laparoscopic cholecystectomy differs little from that for patients scheduled for open cholecystectomy. For patients with a history of peptic ulcer disease, a flexible esophagogastroduodenoscopy (EGD) may be performed to rule out existing disease. For patients with suspected ductal stones, a preliminary ERCP or other diagnostic evaluation is often done. A laparoscopic procedure always has the potential to be converted to a laparotomy—a potential the patient should be informed about before the surgical procedure (Research Highlight). Laparotomy instrumentation and supplies should be available in the OR.
Procedural Considerations.
Patients are generally admitted to the ambulatory surgery center (ASC) on the morning of surgery and will commonly require less than a 24-hour stay or admission to an extended recovery unit (ERU). A general anesthetic is used, and antibiotic prophylaxis may be administered in the immediate preoperative period. The following instrumentation, supplies, and equipment are required for laparoscopic cholecystectomy: laparoscope, two 5-mm trocars and sheaths, two 10- or 11-mm trocars and sheaths (trocar size depends on surgeon preference and may vary), a #7 knife handle with a #11 blade, multiple clip appliers, blunt grasping forceps (an assortment of alligator, Babcock, and spatula), and laparoscopic scissors. A laparoscopic video unit and secondary “slave” monitor, laparoscopic camera and control unit, light source, CO2 source and insufflation unit, ESU, suction-irrigator (disposable), filtered insufflation tubing (disposable), and a pressure bag for IV saline 0.9% are commonly used. Instrumentation and supplies for laparoscopic common bile duct exploration should be available in the room. This may include a balloon-tipped Fogarty catheter, wire baskets, dilators, a T-tube, and a small, flexible choledochoscope. The patient is positioned supine with the usual comfort and safety measures observed. A Foley catheter (for bladder decompression) and an NG tube (for decompression of the stomach) may be inserted. Anesthesia is administered, the time-out completed, and the patient then placed in reverse Trendelenburg position of 10 to 20 degrees.
Pneumoperitoneum may be accomplished using the closed or open technique. In the closed technique, a special hollow insufflation needle (Veress) with a retractable cutting sheath is inserted into the peritoneal cavity through a supraumbilical incision and used for insufflation. In the open technique, sometimes termed the Hasson technique, a small incision is made above or below the umbilicus into the peritoneal cavity. A blunt-tipped cannula (Hasson cannula) with a gas-tight sleeve is inserted, and then insufflation takes place. This approach is used for patients who have had a prior abdominal incision near the umbilicus or for those who have the potential for intraperitoneal adhesions. The Hasson technique may also use sutures, placed on either side of the sleeve, to anchor and hold the sleeve in place.
The gas of choice for pneumoperitoneum is CO2. Gas flow is initiated at 1 to 2 L/minute. Elevated CO2 levels and respiratory acidosis may occur because CO2 diffuses into the patient’s bloodstream during laparoscopy. Intra-abdominal pressure is normally between 8 and 10 mm Hg and the surgeon commonly uses that range as an indicator for proper Veress needle placement. If the pressure gauge shows a higher pressure, the needle may be in a closed space (such as fat), be buried in omentum, or be in the lumen of the intestine. The perioperative nurse should set the insufflation unit to a maximum pressure of 15 mm Hg. When intra-abdominal pressure reaches 15 mm Hg, flow will stop. Pressure higher than 15 mm Hg may result in bradycardia or a change in blood pressure, or it may force a gas embolus into an exposed blood vessel during the operative procedure. Most insufflation units are equipped with an alarm to alert the operative team if the intra-abdominal pressure is exceeded. Alarm systems on clinical equipment should be activated and sufficiently audible with respect to competing noise in the OR. The surgeon may frequently ask what the pressure reading is, as may the anesthesia provider.
Operative Procedure
1. A small skin incision is made in the folds of the umbilicus with a #11 blade on a #7 knife handle.
2. Pneumoperitoneum is created using either the open or the closed technique.
3. An 11-mm trocar is inserted through the supraumbilical incision; this becomes the umbilical port.
5. Three additional trocars are inserted into the peritoneal cavity under direct visualization of the laparoscopic view (Figure 12-8).
6. Blunt grasping forceps are inserted through the medial 5-mm port to grasp the gallbladder.
7. The gallbladder is retracted laterally (Figure 12-9, A), exposing the triangle of Calot. The junction of the gallbladder and cystic duct is then identified. The endoscopic dissector, hook, and scissors are used to partially dissect the base of the gallbladder off the liver bed. Electrosurgery is also used. The electrosurgical instrument (active electrode) may have a channel through which suction can be applied to evacuate smoke plume. Some disposable instruments permit suction, electrocoagulation, and irrigation through the same instrument.
9. An intraoperative cholangiogram may be performed by placing a hemoclip proximally on the cystic duct, incising its anterior surface, and passing the cholangiogram catheter into the duct. Once the cholangiogram is completed, two clips are placed distally on the cystic duct and it is divided (Figure 12-9, B). A pre-tied loop ligature may be used if the duct is large.
10. Attention is then given to dissecting the gallbladder out of its fossa.
11. The surgical site is inspected for hemostasis and the gallbladder dissected off the liver.
12. The gallbladder is then removed through the umbilical port (Figure 12-10). An endobag or similar specimen-retrieval accessory may be used to secure the gallbladder for extraction.
13. The peritoneal cavity is decompressed. The port sites are closed and dressed with Steri-Strips.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree