Surgery for Chronic Mesenteric Ischemia
Mark C. Wyers
Prevalence/Natural History
Atherosclerosis and occlusion of the main visceral trunks, first described by Chienne in 1869, is relatively common affecting <1% to 18% of the general population. Even among peripheral vascular disease patients, however, in whom the incidence may be as high as 27%, symptoms of chronic mesenteric ischemia (CMI) are rare. It is estimated that CMI accounts for <1 hospital admissions per 100,000 patients in the United States. The natural history of asymptomatic mesenteric stenosis is not well known because the clinical diagnosis is difficult to establish. There is good evidence, however, to suggest that isolated stenoses of the celiac or superior mesenteric artery (SMA), if asymptomatic, are likely to remain so and do not confer any significant survival dis-advantage. Asymptomatic patients with
>50% of all three mesenteric vessels have significant chance of developing CMI symptoms and have increased cardiovascular mortality rates. Once symptoms are present, the disease is progressive and can be fatal. Over 40% of the patients presenting with acute mesenteric ischemia have a precedent history of CMI symptoms. Because of the variability of CMI symptoms, referral for treatment may be delayed months to years, after numerous misdirected diagnostic evaluations.
>50% of all three mesenteric vessels have significant chance of developing CMI symptoms and have increased cardiovascular mortality rates. Once symptoms are present, the disease is progressive and can be fatal. Over 40% of the patients presenting with acute mesenteric ischemia have a precedent history of CMI symptoms. Because of the variability of CMI symptoms, referral for treatment may be delayed months to years, after numerous misdirected diagnostic evaluations.
A comprehensive understanding of the normal and variant mesenteric blood supply is important. Redundant collaterals between the main arterial trunks, extrinsic neural and hormonal control, and intrinsic autoregulation of the intestinal microcirculation are responsible for maintaining perfusion until flow rates are critically reduced. Common anatomic variants as well as the potential for any interrupted collateral pathways because of prior bowel resection, aortic procedure, or abdominal surgery must be recognized. It is a common belief that two of the three mesenteric arteries must be involved with significant occlusive disease in order to cause CMI and that the SMA must be one of the two. Isolated celiac or inferior mesenteric artery (IMA) narrowing or occlusion is almost always well tolerated. However, single vessel disease may cause symptoms if there is a critical SMA stenosis or occlusion without adequately developed or interrupted collaterals.
The celiac artery in most cases arises from the aorta at the level of 12th thoracic or first lumbar vertebra. The classic pattern of this artery involves three branching arteries—left gastric, splenic, and common hepatic—configured as a true trifurcation (25%) or, more commonly, with the left gastric branching first (65% to 75%). The next most common variation is the additional presence of a fourth branch, either the dorsal pancreatic or the middle colic artery (5% to 10%). The remaining variations, including a single celiacomesenteric trunk, are much less common, each accounting for <1% of the cases. Hepatic artery anatomy is highly variable beyond its origin with 18% to 20% of hepatic branches originating from the SMA rather than the celiac artery. The common hepatic artery does this with the lowest frequency (2.5%), whereas a replaced or accessory right or left hepatic artery originating from the SMA is relatively common (14% to 18%) and may be clinically significant when considering revascularization strategies. Other less constant, but robust when present, celiac artery–SMA collateral pathways include the Arc of Bühler and Arc of Barkow.
The SMA origin is located approximately at the level of the first lumbar vertebra at a distance of 0.2 to 2 cm inferior to the celiac artery. Typically, the first branch is the inferior pancreaticoduodenal artery, which completes an important collateral pathway with the celiac artery via the superior pancreaticoduodenal and gastroduodenal arteries. The middle colic artery is usually the second SMA branch. It divides subsequently into left and right branches. The former connects with the ascending branch of the right colic artery that arises from a more distal point on the SMA. The latter connects with the ascending branch of the left colic, running immediately adjacent to the splenic flexure to form an important SMA–IMA collateral, the marginal artery of Drummond. This should be distinguished from the meandering mesenteric artery (Arc of Riolan) that courses radially through the mid portion of the mesenteric arcade, near the inferior mesenteric vein. A variable number (10 to 15) of jejunal and ileal branches originate from the left side of the SMA as it courses toward the right lower quadrant.
The IMA typically arises from the left anterolateral aspect of the aorta at the level of the third lumbar vertebra and divides into the left colic and two or three sigmoidal branches. The ascending left colic branch forms the lower portion of the marginal artery of Drummond. The terminal branches of the sigmoidal arteries form the left and right superior rectal arteries that complete collateral pathways to the internal iliac arteries via the middle rectal and internal pudendal arteries.
Clinical Presentation
The clinical diagnosis of CMI can be difficult and is frequently delayed. General atherosclerotic risk factors contributing to visceral atherosclerosis are often present, but other rare associations with neurofibromatosis, fibromuscular dysplasia, rheumatoid arthritis, and polyarteritis have also been reported. Patients typically present in their 60s and 70s and are more often female. The sine-qua-non of CMI combines postprandial abdominal pain with unintentional weight loss. This classic pain syndrome involves dull, achy epigastric discomfort, begins within 15 to 30 minutes of a meal and can last hours. Transient nausea or emesis soon after eating is also not uncommon. In response to this, patients can develop so-called “food fear” and many learn (consciously or unconsciously) to modify their eating habits to minimize discomfort. Careful dietary history may reveal a tendency to avoid calorie-rich foods and smaller, more frequent meals. Bowel habits may or may not be abnormal and may include either constipation or diarrhea.
Abdominal exam is frequently unrevealing. The patient may or may not appear cachectic. Pain is often reported as episodic without significant or localizable tenderness. If pain is persistent or tenderness is present, this may indicate a subacute presentation and should be treated expeditiously. Laboratory evaluation is also frequently unremarkable but may support malnutrition; in more acute presentations, leukocytosis or dehydration may be present. Lactic acidosis is a late finding in acute presentations.
CMI is largely a diagnosis of exclusion and it is important to rule out other, more common causes of abdominal pain and weight loss. Many of these have already been done prior to being referred to a vascular surgeon. Upper and lower endoscopies are typically negative. Abdominal CT may show arterial calcifications but is otherwise negative. General cancer screening is negative. The workup is tailored depending on the overall clinical suspicion generated by the patient history. If there is access to good quality mesenteric duplex, this is a very helpful, inexpensive, and non-invasive screening test. CT scanning is fairly ubiquitous, but a high-quality CT angiography is extremely valuable and should be obtained in almost every case prior to proceeding with mesenteric arteriography.
Imaging
Duplex Ultrasound
Duplex ultrasonography accurately identifies high-grade stenoses of the celiac artery and SMA. It is the non-invasive diagnostic study of choice in the patients with symptoms suggesting CMI. However, like most specialized duplex applications, there is a significant amount of expertise required of the vascular technologist. Careful attention to proper angle correction is crucial to avoid falsely elevated velocities. In addition, to minimize interference from overlying bowel gas, the study is best performed after an overnight fast. Pre-scan dosing of simethicone may also improve visualization.
Authors from the Oregon Health Sciences University and from Dartmouth were the first to propose duplex criteria for the diagnosis of splanchnic artery stenosis or
occlusion in 1991. Both groups have since published validation studies for their criteria, and the diagnostic thresholds have been published for peak systolic velocity (PSV) and end-diastolic velocity (EDV). For the SMA, a PSV >275 cm/s demonstrated a sensitivity of 92% and a specificity of 96% for a >70% angiographic stenosis. A EDV of >45 cm/s had a sensitivity of 90% and a specificity of 91% for a stenosis >50%. For the celiac artery, a PSV >200 cm/s demonstrated sensitivity of 90% and specificity of 91% for a stenosis of >70%. Celiac EDV >55 cm/s had a 93% sensitivity and 100% specificity for a >50% stenosis. Retrograde hepatic artery flow is 100% predictive of a severe celiac artery stenosis or occlusion.
occlusion in 1991. Both groups have since published validation studies for their criteria, and the diagnostic thresholds have been published for peak systolic velocity (PSV) and end-diastolic velocity (EDV). For the SMA, a PSV >275 cm/s demonstrated a sensitivity of 92% and a specificity of 96% for a >70% angiographic stenosis. A EDV of >45 cm/s had a sensitivity of 90% and a specificity of 91% for a stenosis >50%. For the celiac artery, a PSV >200 cm/s demonstrated sensitivity of 90% and specificity of 91% for a stenosis of >70%. Celiac EDV >55 cm/s had a 93% sensitivity and 100% specificity for a >50% stenosis. Retrograde hepatic artery flow is 100% predictive of a severe celiac artery stenosis or occlusion.
Ct Angiography
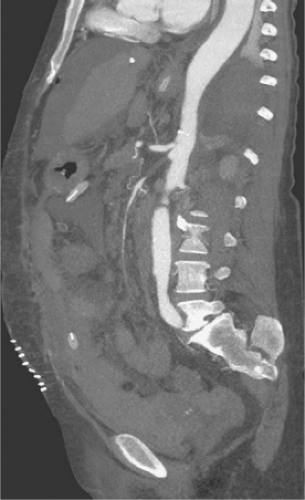
Several authors have described the use of ultrafast multidetector row CT arteriography for the evaluation of both acute mesenteric ischemia and CMA. The widespread availability of these newest generation CT scanners represents a potential change in the diagnostic algorithm for the workup of mesenteric ischemia and offers the advantage of speed compared to angiography. If a high-resolution multidetector CT arteriogram (CTA) is performed, a significant amount of information can be obtained about the central arterial and venous circulation. Accurate timing of contrast injection and fine slices through the upper abdomen usually provide excellent visualization of the celiac artery and SMA. CT also offers the ability to exclude other causes of abdominal pain and some ability to assess bowel perfusion. The exact timing of intravenous contrast is tailored to the specific clinical question. The use of traditional oral “positive” contrast agents detracts from image quality, and most visceral CTA protocols recommend the use of a “negative” oral contrast agent such as water (500 to 750 cc) given immediately before the scan. This prevents image artifact from pooled areas of high opacification within the intestinal tract and also enhances the ability to see bowel wall enhancement (or lack thereof) in the late arterial phase of the contrast bolus. Main branches of the celiac and SMA are seen remarkably well using multidetector CTA (MDCTA) because of thinner collimation (0.5 to 1.5 mm) and overlapping data acquisition. This reduces the amount of volume averaging and creates higher quality 3D volume sets for reformatting and interpretation (Fig. 1). The same scan is used to detect arterial narrowings or occlusions and associated bowel abnormalities such as wall thickening, pneumatosis, or poor mucosal enhancement that support the diagnosis of more acute or subacute presentations of mesenteric ischemia. In a recent study by Kirkpatrick et al., CT angiographic visualization was judged to be satisfactory in all cases up to second-order branches of both the celiac artery and SMA. Angiography was available in only three patients but correlated well with the CTA findings. In practice, the presence or absence of collateral pathways can also be seen with high-quality CTA.
Biplanar Arteriography
Traditional multiplanar aortography remains the definitive diagnostic study for mesenteric ischemia and offers the opportunity for percutaneous treatment via angioplasty and stenting. Though CTA gives adequate images to confirm the proximal mesenteric anatomy, they are static. Angiography remains important for diagnostic imaging and preoperative planning based on superior image resolution, ability to visualize collateral flow direction, and identification of disease in the distal portions of the splanchnic arterial bed. Angiography, with selective injections of any patent vessels, allows a more quantitative assessment of collaterals. The rapidity, number, and size of vessels help greatly to determine the essential requirements for revascularization. For example, a flush SMA occlusion that would otherwise require surgical bypass could be left alone in favor of stenting a critical celiac stenosis with good pancreaticoduodenal collaterals. More importantly, in the endovascular era, angiography is an immediate segway to patient treatment.
Magnetic Resonance (Mr) Angiography
Magnetic resonance (MR) imaging of the splanchnic vessels is an evolving technology. It is theoretically appealing because it is noninvasive, avoids the risk of allergic reaction and nephrotoxicity associated with iodinated contrast agents, and may not be as operator-dependant as duplex ultrasound. Unlike other modalities, MR has the theoretical ability to incorporate both functional and anatomic evaluations of the mesenteric circulation. Functional assessment is not yet used clinically but has been investigated by several groups using non-contrast, cine cardiac-gated phase-contrast (PC) MRA to correlate superior mesenteric venous (SMV) and SMA flow rates. Burkart et al. showed that patients with CMI (n = 10) had a reduced rate of postprandial flow augmentation (64 ± 28%; P = 0.02) as compared to healthy controls (n = 10). This type of analysis may provide physiologic information to confirm the diagnosis of CMI suspected clinically. Furthermore, pre- and postprandial comparisons in human subjects may provide the ability to distinguish the overall adequacy of arterial blood flow. Li et al. were successful at distinguishing patients with CMI from those without using only SMV blood T2 measurements. In healthy patients, the postprandial SMV T2 measurements increased compared to fasting, whereas the same measure decreased in symptomatic CMI patients (p < 0.0001).
Anatomic imaging of the visceral vessels relies on contrast-enhanced MR techniques; non-contrast MRA is much less accurate. Rapid bolus intravenous administration of a T1-shortening agent such as gadolinium DTPA paired with a rapid, 3D gradient-recalled echo sequence allows consistent imaging of the splanchnic circulation with minimal flow artifact. Commercially available data acquisition protocols are available and the 3D dataset can be post-processed with techniques such as maximal intensity projection, curved planar re-formation, and volume rendering, which distill the data into more readily comprehensible images. Two studies recently addressed the accuracy of 3D contrast-enhanced MRA in evaluating
percent stenosis in the celiac artery, SMA, and IMA. The most common error was overestimation of the stenosis. The weakness of MRA is its relatively poor spatial resolution that, even on the best systems, is limited to 1 mm. Gadolinium-enhanced MRA does not currently provide sufficient resolution to demonstrate distal emboli; non-occlusive, low flow states; small vessel occlusion; or vasculitis. Meaney et al. evaluated 14 patients with CMI; MRA had a sensitivity of 100% and a specificity of 87% in the overall detection of visceral artery stenosis ≥50%. The lack of specificity was due to the false positive diagnosis of IMA stenoses. Looking only at the subset of celiac and SMA data, the sensitivity and specificity were both 100% for the detection of stenosis ≥50%. In a similar and more recent study by Carlos et al., two blinded observers reviewed gadolinium-enhanced MR angiograms and compared them to conventional arteriogram in 26 patients suspected to have CMI. The overall accuracies for the detection of stenosis ≥50% or occlusion in the celiac, SMA, and/or IMA were 95% and 97%. Secondary signs of mesenteric ischemia such as indurated fat or bowel wall thickening, which are routinely delineated by CT, are more difficult to assess with MR. In general, however, the anatomic evaluation of the mesenteric arteries is limited to the proximal celiac and SMA only and the evaluation of SMA branches or IMA is very limited by the spatial resolution of MR imaging techniques.
percent stenosis in the celiac artery, SMA, and IMA. The most common error was overestimation of the stenosis. The weakness of MRA is its relatively poor spatial resolution that, even on the best systems, is limited to 1 mm. Gadolinium-enhanced MRA does not currently provide sufficient resolution to demonstrate distal emboli; non-occlusive, low flow states; small vessel occlusion; or vasculitis. Meaney et al. evaluated 14 patients with CMI; MRA had a sensitivity of 100% and a specificity of 87% in the overall detection of visceral artery stenosis ≥50%. The lack of specificity was due to the false positive diagnosis of IMA stenoses. Looking only at the subset of celiac and SMA data, the sensitivity and specificity were both 100% for the detection of stenosis ≥50%. In a similar and more recent study by Carlos et al., two blinded observers reviewed gadolinium-enhanced MR angiograms and compared them to conventional arteriogram in 26 patients suspected to have CMI. The overall accuracies for the detection of stenosis ≥50% or occlusion in the celiac, SMA, and/or IMA were 95% and 97%. Secondary signs of mesenteric ischemia such as indurated fat or bowel wall thickening, which are routinely delineated by CT, are more difficult to assess with MR. In general, however, the anatomic evaluation of the mesenteric arteries is limited to the proximal celiac and SMA only and the evaluation of SMA branches or IMA is very limited by the spatial resolution of MR imaging techniques.
CMI, in early reports, was likened to angina and was treated with trinitrin. Modern treatment goals are to reestablish adequate circulation to prevent bowel infarction, relieve symptoms, and restore normal nutrition and weight. Early revascularization attempts involved local endarterectomy, but it was the textile revolution about the same time that gave us the first polyester bypass grafts. Various configurations of these grafts remain important in the treatment of CMI and have long-term durability. Endovascular therapy in the last decade has again revolutionized the treatment of these challenging patients. Overall treatments for mesenteric ischemia have been increasing significantly over the recent decade; endovascular treatments in the United States surpassed operative bypass in 2002.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree