Supravesical Urinary Diversion
Badrinath Konety
Sean P. Elliott
Introduction
Supravesical diversion involves the external diversion of the urine stream without use of the bladder. While percutaneous nephrostomy offers a short-term alternative to operative diversion, problems with frequent tube changes, infection, and external appliances make surgical diversion the preferred method in those needing long-term supravesical diversion. This chapter focuses on surgical supravesical diversion. Except in the case of cutaneous pyelostomy or ureterostomy, supravesical diversion employs intestinal segments to bridge the gap between the ureters and the skin. Types of supravesical diversion may generally be categorized as continent and noncontinent. Noncontinent diversions involve a wide stoma and an external appliance to collect the urine (urostomy bag). Heterotopic continent diversions utilize a catheterizable stoma on the abdominal wall to empty an intra-abdominal neobladder whereas orthotopic continent diversions create a pelvic neobladder that is anastomosed to the urethra.
The most common indication for supravesical diversion is following radical cystectomy for cancer of the bladder. Other indications include refractory ureteral or urethral obstruction and urinary fistula: these are often less amenable to repair when the etiology is radiation damage. Radiation cystitis, interstitial cystitis, or hemorrhagic cystitis not responsive to less invasive means of management are managed with supravesical diversion. Additionally, supravesical diversion may be considered in the management of neurogenic bladder when bladder augmentation cystoplasty is not an option.
Patients should be appropriately selected to ensure they are medically fit for major surgery. Coexistent bowel pathology including inflammatory bowel disease or previous resection should factor into selection of the appropriate intestinal segment for diversion.
Patient considerations specific to the choice of the type of urinary diversion include visual, cognitive, neuromotor, and renal function as well as the level of available social support. As continent neobladders can be associated with electrolyte disturbances they are to be avoided in patients with cognitive or renal impairment. When considering a neobladder, one must take into account the patient’s neuromotor status and dexterity along with the availability of other caregivers to assist with care of the diversion. Patients can suffer from urinary retention or incomplete emptying after an orthotopic neobladder and hence the ability to catheterize the neobladder is important in them as well as in those considering a heterotopic neobladder with an abdominal stoma. When the ability to catheterize or electrolyte disturbances are a concern, the preferred diversion is one with a noncontinent stoma.
If a urinary diversion is being contemplated in a patient with ureteral stricture, it is important to delineate the length and location of the stricture before surgery. As an indwelling stent may mask the true extent of disease, a preoperative nephrostomy and ureteral stent removal is recommended. An antegrade ureterogram several weeks later or in the operating room at the time of reconstruction will best identify the level for ureteral transection. A stent-free period also allows ureteral inflammation associated with the stent to resolve before reconstruction. Because such inflammation can make intraoperative identification of the ureters more difficult due to periureteral scarring and induration of the plane inside Gerota’s fascia, antegrade ureteral catheters may be placed down to the site of obstruction on the day of diversion.
It is always advisable to remove the bladder when performing supravesical diversion, even in the absence of bladder malignancy. Diversion of the urine stream away from an intact bladder too frequently results in pyocystis, a condition of retained mucous and desquamated bladder epithelial cells complicated by infection. Pyocystis is managed by bladder drainage and/or cystectomy. One alternative in women is to create an iatrogenic fistula into the vagina, which allows for bladder drainage without the need for an indwelling or intermittent catheterization.
When anastomosing the ureters to the intestinal segment one may elect a tunneled or a refluxing reimplant. Longitudinal studies have shown the risk of renal dysfunction from obstruction in a tunneled reimplant may outweigh the risk of renal dysfunction from reflux in a nontunneled, refluxing reimplant. Most opt for a refluxing reimplant in the modern era. Refluxing reimplants may be done in the fashion of Bricker or Wallace. The Bricker reimplant is the technique of separately anastomosing the two ureters to the bowel in an end-to-side manner. The Wallace reimplant is the technique of a side-to-side anastomosis of the two ureters to each other followed by an end-to-end anastomosis of the “double-barrel” ureters to the back end of a loop of intestine. The Wallace anastomosis makes it easier to endoscopically access the ureters postoperatively if there is a need for upper tract evaluation or stone removal. The principal concern about the Wallace anastomosis is that obstruction of the terminal portion of one ureter, due to stenosis, a stone, or recurrent tumor, may obstruct both ureters, resulting in critical renal impairment. If a ureteric or renal pelvic tumor should develop in one renal moiety, it would be harder to separately dissect out the corresponding ureter at the time of a nephroureterectomy. Tunneling techniques are covered below in the relevant sections.
Noncontinent Diversions
Cutaneous Ureterostomy
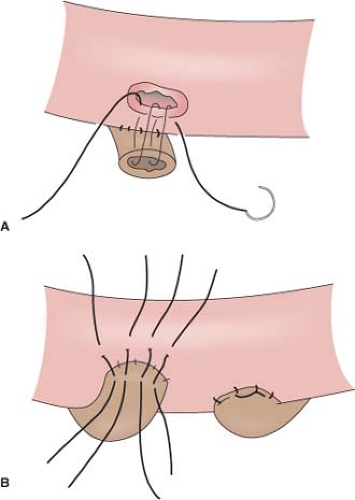
Cutaneous loop ureterostomy or pyelostomy can be performed as a temporary diversion to manage the infant with severe hydroureteronephrosis in anticipation of future reimplant. Due to the high rate of stomal stenosis, few employ cutaneous end ureterostomy as a permanent diversion for adults in the modern era. The degree of mobilization of the ureters that is required in order to bring them to skin results in devascularization and stenosis in approximately 60%. The small caliber ureter may also be predisposed to stomal stenosis. However, in the patient with multiple comorbidities and very dilated ureters, there may still be a role for permanent cutaneous ureterostomy. In order to avoid two stomas it is customary to perform a “double-barrel” ureterostomy. Stomal stenosis may be minimized by eversion of the ureters into a rosebud and by incorporating a V-shaped skin flap into each ureterostomy.
As the two skin flaps are brought in from different directions on the two ureters of the “double barrel,” the result is a Z-plasty.
As the two skin flaps are brought in from different directions on the two ureters of the “double barrel,” the result is a Z-plasty.
Ileal Conduit
Originally pioneered by Bricker in 1950, the ileal conduit is the most commonly performed type of supravesical urinary diversion. Like other ureterointestinal diversions, ureteral devascularization and the risk of stomal stenosis is minimized because the intestine is brought to the ureters rather than mobilizing the ureters to the skin. Contraindications include short bowel, radiation enteritis, and Crohn’s disease. After transecting the ureters at the appropriate level, holding sutures are placed on the ureters and a 15- to 20-cm segment of terminal ileum is selected. Vitamin B12 and folate malabsorption is minimized by selecting a segment at least 20 cm proximal to the ileocecal valve. Good blood supply to the ileal segment may be confirmed by palpation or backlighting of the mesentery. After identifying an adequate pedicle, the appropriate length segment of ileum can be marked with sutures on the bowel. The avascular portion of the mesentery is then divided proximally and distally to create windows, between ligatures or with electrocautery. The ileum is divided, usually with a GIA stapler. The ileal segment is dropped toward the pelvis and the remaining bowel is brought back into continuity with a two-layer sutured anastomosis or a stapled side-to-side anastomosis. The mesenteric defect is closed to prevent internal hernias. Due to the risk of urinary stones forming on the staple line, some prefer to exclude the staple line from the proximal end of the conduit using an absorbable suture in a horizontal mattress. One can open the distal/cutaneous end of the conduit at this time and irrigate the interior.
A window is created either behind or through the sigmoid mesentery to allow passage of the left ureter to the right retroperitoneum in front of the sacral promontory. It is important to do this under direct vision in order to avoid injuring the presacral veins—controlling bleeding from these veins is difficult. The tunnel should be wide enough to prevent kinking of the ureter and care must be taken to avoid twisting of the ureter. The ureter is brought across using the previously placed holding suture.
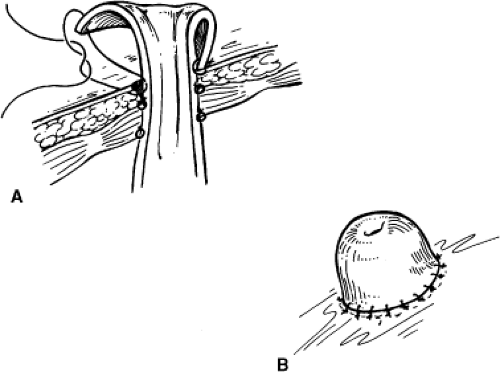
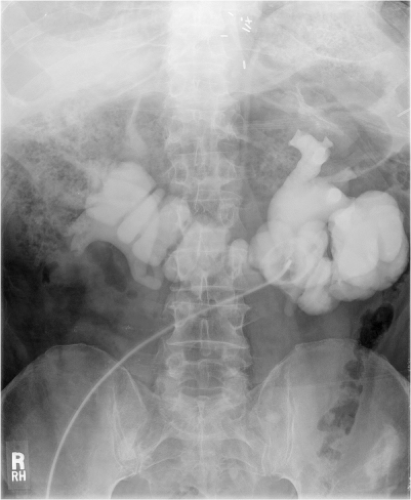
It cannot be overemphasized that ureteral mobilization should be kept to a minimum and the intestinal segment should be brought to the retroperitoneum, not the ureters into the peritoneum. If performing a standard Bricker ureterointestinal anastomosis, a small enterotomy is made on the posterior aspect of the proximal end of the ileal conduit. We find it easiest if a seromuscular suture is used to hold up on the intestine and the enterotomy made with scissors just below this. The bleeding from the mucosal edges makes visualization difficult but cautery should be avoided in order to preserve blood supply at the anastomosis site. Some place mucosal eversion stitches, with fine absorbable suture—these control bleeding nicely and make identification of the mucosa during the anastomosis straightforward. Others avoid extra sutures in order to minimize inflammation that may lead to subsequent scarring. The ureter is spatulated and a running or interrupted mucosa-to-mucosa anastomosis is performed with fine absorbable monofilament suture (Fig. 1). After one side of the ureter is completed a “single J” ureteral stent is placed. The coil end of the stent is placed through the open ureter up to the kidney over a guidewire, about 15 to 20 cm. Correct placement can be confirmed with a postoperative plain film of the abdomen. A Schnidt clamp is passed from the cutaneous end of the conduit up to anastomosis to grasp the straight end of the stent and bring it out from the end of the conduit. An absorbable suture is used to secure the stent to the distal bowel. The second half of the ureter is closed and the same is repeated on the other side.
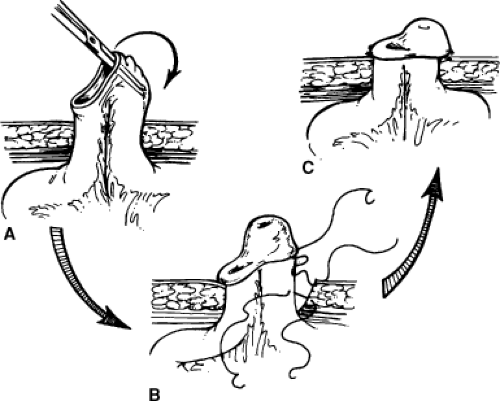
In a Wallace anastomosis, the staple line is trimmed off the proximal end of the conduit. The ureters are spatulated medially and the posterior walls are sewn to each other in a running or interrupted fashion with fine absorbable suture (Fig. 2). The ureters are then sewn to the proximal open end of the conduit. We prefer to sew the joined apical ends of the ureters to the bowel first as this can be the most difficult portion. The anterior walls and distal ends of the joined ureters can then be sewn to the bowel. Ureteral stents are placed before completing the anastomosis, as previously described.
A site on the skin for the urostomy should be previously marked after consultation with a stoma therapist. A disk of skin is excised. The underlying column of fat is removed down to the rectus fascia. A cruciate incision is made in the anterior rectus fascia and the rectus muscles separated. A fasciotomy is then made in the posterior sheath. The fascial defect should accept two fingers. Then, 2-0 or 3-0 absorbable sutures are placed in the outer portions of the cruciate incision and the needles left on. A Babcock clamp is used to grasp the conduit and stent and bring them through the fascia. The conduit is then anchored to the fascia using the previously placed 2-0 or 3-0
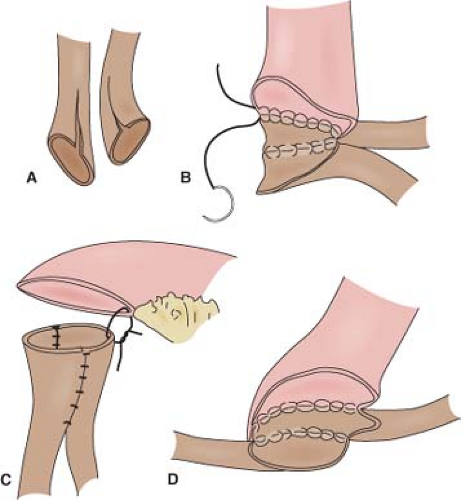
sutures. Bricker originally described a stoma that was flush with the skin. Brooke advocated use of the everted “rosebud” stoma, which helps prevent stomal stenosis and minimizes urinary skin irritation by ensuring that the effluent urine drips directly into the bag. This is achieved with three or four stitches of a 3-0 absorbable suture passed from the skin to the deep portion of the conduit close to the fascia and then through the distal end of the conduit (Fig. 3). When tying these down, a Babcock clamp can be used to grasp the inner mucosa and pulled up to encourage eversion. The rest of the anastomosis of conduit to the skin can then be done with absorbable stitches. The back end of the loop is anchored by sewing the cut edge of posterior peritoneum over it with three to five interrupted silk sutures. This secures the conduit at both ends and prevents volvulus as well as retroperitonealizing the conduit. This can contain potential urine leaks in the retroperitoneum.
sutures. Bricker originally described a stoma that was flush with the skin. Brooke advocated use of the everted “rosebud” stoma, which helps prevent stomal stenosis and minimizes urinary skin irritation by ensuring that the effluent urine drips directly into the bag. This is achieved with three or four stitches of a 3-0 absorbable suture passed from the skin to the deep portion of the conduit close to the fascia and then through the distal end of the conduit (Fig. 3). When tying these down, a Babcock clamp can be used to grasp the inner mucosa and pulled up to encourage eversion. The rest of the anastomosis of conduit to the skin can then be done with absorbable stitches. The back end of the loop is anchored by sewing the cut edge of posterior peritoneum over it with three to five interrupted silk sutures. This secures the conduit at both ends and prevents volvulus as well as retroperitonealizing the conduit. This can contain potential urine leaks in the retroperitoneum.
An alternative to the Bricker stoma is a Turnbull loop stoma (Fig. 4). This may reduce stomal stenosis because blood supply to the stoma is not compromised as it is with the terminal Bricker stoma. The Turnbull stoma is also advantageous in the obese patient with thick mesentery in whom it can be difficult to get the mesentery to reach the abdominal wall with a Bricker stoma. A shortcoming of the Turnbull stoma is its higher risk of parastomal hernia due to the necessarily larger fascial defect. After bringing a loop of ileum through the fascial defect and securing it to the fascia, the antimesenteric side is opened and the mucosa is matured to the deep portion of the loop just above the fascia and then to the skin, as for the Brooke modification of the Bricker stoma. One can opt to pass a red rubber catheter or a loop colostomy rod through a window in the mesentery below the loop and suture it to the skin in order to prevent recession of the stoma in the immediate postoperative period. Because the edema that sometimes occurs postoperatively can cause this catheter to erode the skin, an alternative is to simply suture the catheter to itself off the abdominal wall to complete a circle. This can be removed about 2 weeks later.
Colon Conduit
The ileal conduit is familiar to most urologists. However, one should be prepared to perform a colon conduit in select patients. When total pelvic exenteration is performed, the sigmoid colon conduit is advantageous in that it avoids a bowel anastomosis. A transverse colon conduit is essential in cases of long segment ureteral stricture or radiation ureteritis.
After identifying an adequate pedicle, the necessary length of sigmoid colon is selected. It should be noted that in some older patients the sigmoid colon can be redundant due to long-standing constipation. Unlike the ileal conduit, it is not necessary to pass the left ureter across to the right side. Once the sigmoid colon mesentery is mobilized the two ureters can be brought toward the midline or the colon can be stretched across the retroperitoneum. Anastomotic techniques are as described below for the transverse colon conduit.
Because the transverse colon and its middle colic artery originate in the upper abdomen this segment can be anastomosed to the upper one-third of the ureter or even directly to the renal pelvis (Fig. 5). The transverse colon has the added advantage of being out of the pelvic radiation field whereas the ileum may be affected by radiation enteritis.
The combination of unirradiated bowel and unirradiated ureters and the absence of ureteral mobilization optimizes success in the high-risk radiation patient.
The combination of unirradiated bowel and unirradiated ureters and the absence of ureteral mobilization optimizes success in the high-risk radiation patient.
It is important to mobilize the hepatic and splenic flexures of the colon. For this reason it is frequently necessary to extend the abdominal incision to the xiphoid. The middle colic artery is easily identified. The entire transverse colon is isolated and divided as described above for the ileal conduit. While the segment will seem long, the length is necessary in order to reach both upper ureters as no ureteral mobilization will be done and the left ureter won’t be brought to the right side as is done in the ileal conduit. Depending on the preference of the surgeon, the transverse colon can be brought intraperitoneal or extraperitoneal. If intraperitoneal, the colon is brought into continuity above the colon conduit, as described for the ileal conduit. The ureters are brought through windows in the left and right colonic mesentery, avoiding injury to the inferior mesenteric vein on the left side. Small enterotomies are made in the portion of the colon adjacent to each ureter. We avoid the tenia coli when performing refluxing anastomosis. In this case, the mucosa is matured, the anastomosis is performed, and stents are placed as described for the ileal conduit earlier. Because tunneled anastomoses are usually reserved for cases in which fecal contamination raises concern for pyelonephritis, we will reserve discussion of these techniques until the section on ureterosigmoidostomy.
Because the intraperitoneal approach to the transverse colon conduit can be difficult in a patient with (a) very short ureters or a planned anastomosis to the renal pelvis, (b) a thick fatty colonic mesentery, or (c) an edematous mesentery after a long operation, we frequently prefer the alternative retroperitoneal approach. In this case the bowel is brought into continuity below the conduit and the conduit is stretched across the retroperitoneum, from kidney to kidney. The upper ureters or renal pelvis can then be anastomosed to the bowel as described above. Regardless of the approach, bringing the stents through the terminal end of the conduit can be difficult due to the long length of the conduit, relative to an ileal conduit. This sometimes requires using a flexible cystoscope to pass a wire first or making a small colotomy midway through the conduit to help guide the stents through with a long clamp.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree