Supraaortic Endovascular Revascularization
Daniel Clair
Samir Shah
Introduction
Disease of the supraaortic trunks—the brachiocephalic, left common carotid, and left subclavian arteries—is uncommon. Studies place the incidence of brachiocephalic arterial occlusive lesions as only 0.5% to 2% of all vascular lesions. Distal carotid disease, in contrast, accounts for 25% of all ischemic cerebrovascular accidents, which collectively is the third leading cause of death in the United States.
The treatments of both disease entities, however, have undergone substantial changes since the advent of endovascular technology. Indeed, supraaortic trunk disease is now routinely treated first with balloon angioplasty and stent placement rather than extrathoracic bypass, vessel transposition, and endarterectomy, which were the staples of conventional revascularization strategies. Similarly, although carotid endarterectomy (CEA) is still the foundation of intervention for carotid arterial disease, the evidence supporting the use of carotid artery stenting (CAS) in select populations has been accruing since the first carotid angioplasty in 1979.
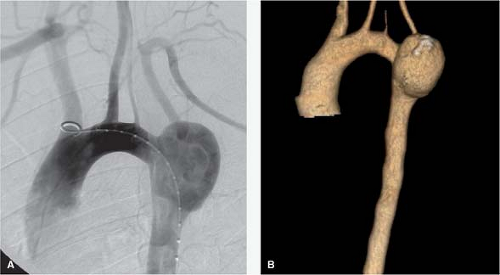
Although anatomic variations of the supraaortic trunks may not have physiologic consequences, they are critical to the formulation of an endovascular strategy. Reviews of CT angiograms have reported that about two-thirds of individuals have the standard aortic arch branching pattern, consisting of a proximal brachiocephalic artery giving rise to the right subclavian and right common carotid arteries, followed by the left common carotid and left subclavian arteries originating distally directly from the aortic arch. The so-called bovine arch, in which the left common carotid shares an origin with the brachiocephalic artery, is the most common anatomic variant, occurring in one-fourth of patients. The second most common variant is direct origination of the left vertebral artery, typically proximal to the left subclavian artery, in 1 of 20 patients (Fig. 1). The most frequent anatomic anomaly noted in this region is an aberrant right subclavian artery originating distal to the left subclavian artery, which occurs about 1% of the time. Numerous other rarer variants and anomalies have been reported.
Carotid anatomy, in contradistinction, is not as variable in its basic elements, but its specific features and morphology—for example, degree of tortuosity and presence of tandem common carotid lesions—heavily influence the feasibility and appropriateness of angioplasty and stenting, as discussed below.
The traditional indication for treatment of supraaortic trunk occlusive disease is the presence of symptoms. Although intervention for asymptomatic stenosis has been reported, the treatment of these lesions is more commonly reserved for symptomatic disease. The principal etiology is atherosclerotic disease, followed by Takayasu’s arteritis, radiation endarteritis, and fibromuscular dysplasia. Other rare causes have also been noted. Brachiocephalic lesions may produce right upper extremity claudication, vertebrobasilar insufficiency, or right cerebral stroke. Left common carotid disease may produce left hemispheric stroke, while lesions of the left subclavian artery may produce either vertebrobasilar insufficiency via a steal phenomenon or left upper extremity claudication. Patients with in situ internal mammary artery–coronary artery bypass grafts are at risk for coronary steal phenomena. Indeed, this was the most common indication for endovascular intervention in 83 consecutive patients at Cleveland Clinic (Table 1).
Carotid disease becomes symptomatic via stroke or transient ischemic attack, a focal reversible neurologic deficit, or amaurosis fugax. Treatment is indicated in the setting of high-grade, cervical carotid stenosis with symptoms. The threshold for intervention in
asymptomatic stenosis is less clear, although data from the Asymptomatic Carotid Atherosclerosis Study (ACAS) and European Carotid Surgery Trial (ECST) favor surgical revascularization compared with medical management for these patients as well. In general, therapy is recommended for asymptomatic patients with >80% carotid artery stenosis.
asymptomatic stenosis is less clear, although data from the Asymptomatic Carotid Atherosclerosis Study (ACAS) and European Carotid Surgery Trial (ECST) favor surgical revascularization compared with medical management for these patients as well. In general, therapy is recommended for asymptomatic patients with >80% carotid artery stenosis.
Table 1 Indications for Supraaortic Trunk Endovascular Intervention in 83 Consecutive Patients | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 2 Features Associated with Higher Risk for Carotid Endarterectomy | |
|---|---|
|
Patient selection for CAS is somewhat controversial. Several prospective randomized trials have compared CAS and CEA with varying results. The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial examined 334 high-risk patients with either asymptomatic stenosis of at least 80% or symptomatic stenosis of at least 50% and concluded that stenting was not inferior to endarterectomy. While only 12.2% of stented patients had major adverse events at 1 year versus 20.1% in the endarterectomy group (P = 0.053), the majority of these differences were driven not by neurologic events, but by coronary events. Similarly, the CArotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) trial randomized 504 low-to-moderate risk symptomatic patients to either CEA or carotid intervention and found no difference in the incidence of death or stroke at 30 days and at 3 years. The Stent protected Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE) trial randomized symptomatic patients between CEA and carotid stenting, but was halted because of futility in attempting to prove the noninferiority of CAS versus CEA. In contrast, the Endarterectomy versus Angioplasty in patients with Symptomatic Severe carotid Stenosis (EVA-3S) trial reported superiority of endarterectomy with respect to stroke or death within 30 days and at 4 years in a population with symptomatic stenosis ≥60%. There are concerns regarding the methods in all of these trials and the ultimate conclusions are suspect according to advocates of both interventional and surgical approaches.
Currently, CAS should be limited to patients at high risk for CEA, either secondary to medical comorbidities or anatomic reasons (Table 2). Qualifying medical conditions include severe cardiopulmonary disease (e.g., recent myocardial infarction, ejection fraction <30%, and multivessel coronary artery disease), renal insufficiency, and age >80 years. Anatomic features conferring higher risk for endarterectomy include contralateral carotid artery occlusion, contralateral laryngeal nerve palsy, prior ipsilateral CEA, prior cervical radiation or surgery, and a high cervical or thoracic carotid lesion.
Table 3 Relative and Absolute Contraindications to CAS | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Contraindications to CAS may similarly be classified as medical or anatomic (Table 3, Fig. 2). It is known that patients older than 79 years and those with symptomatic disease, intracranial microvascular disease, renal insufficiency, severe aortic valvular stenosis,
nd stroke within 6 weeks are at higher risk for CAS. One will recognize that there are some similar medical risk factors for CEA and CAS. Anatomic reasons relatively contraindicating CAS include poor femoral access, diseased or tortuous aortic arch or common carotid artery, diseased or tapering distal internal carotid artery, and long subtotal internal carotid occlusion or external carotid occlusion. Absolute contraindications include circumferential carotid calcification, chronic internal carotid occlusion, intraluminal thrombus, the inability to take clopidogrel or aspirin, and the presence of an intracranial aneurysm or arteriovenous malformation requiring treatment.
nd stroke within 6 weeks are at higher risk for CAS. One will recognize that there are some similar medical risk factors for CEA and CAS. Anatomic reasons relatively contraindicating CAS include poor femoral access, diseased or tortuous aortic arch or common carotid artery, diseased or tapering distal internal carotid artery, and long subtotal internal carotid occlusion or external carotid occlusion. Absolute contraindications include circumferential carotid calcification, chronic internal carotid occlusion, intraluminal thrombus, the inability to take clopidogrel or aspirin, and the presence of an intracranial aneurysm or arteriovenous malformation requiring treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree