Superior Mesenteric Artery Embolectomy and Bypass for Acute Mesenteric Ischemia
C. Keith Ozaki
Edward Kelly
Management of the patient facing life-threatening acute mesenteric ischemia maximally challenges the intellectual, technical, clinical, and team leadership skills of the surgeon. Several pathologic mechanisms can result in acute mesenteric ischemic syndromes, including embolism to the superior mesenteric artery (SMA), mesenteric arterial thrombosis (e.g., acute thrombosis of a chronic SMA atherosclerotic lesion), arterial dissections, low-flow nonocclusive mesenteric ischemia (usually with splanchnic vasoconstriction), and mesenteric venous thrombosis. The heart stands as the most common source for emboli, and cardiac pathologies that predispose to thrombus formation include atrial fibrillation (especially in patients with anatomic abnormalities such as atrial dilation who are not anticoagulated or are underanticoagulated), recent myocardial infarction with mural thrombus formation, and ventricular aneurysm. The following discussion focuses on SMA thromboembolism and acute-on-chronic thrombosis, which together account for the majority (,75%) of acute mesenteric ischemia cases.
Acute mesenteric ischemia remains a highly lethal clinical event, with mortality averaging around 70% from several series. While uncommon, the incidence of this problem may be increasing in the aging population and with the rising incidence of the metabolic syndrome, defined as the accumulation of atherogenic risk factors (e.g., obesity, dyslipidemia, hypertension, glucose intolerance), resulting in a prothrombotic and proinflammatory state. It is also possible that the contribution of the metabolic syndrome to the incidence of acute mesenteric ischemia may be offset by increasing use of anticoagulation for other diagnoses, such as dysrhythmias. A recent population-based series cites an incidence rate as high as 5.3 cases per 100,000 inhabitants per year.
Anatomically the SMA arises from the anterior aorta typically 1 to 2 cm below the celiac artery, and 1 to 2 cm cephalad to the takeoff of the renal arteries. It usually originates at the level of the first lumbar vertebral body and passes inferiorly and to the right, posterior to the pancreas, and anterior to the fourth portion of the duodenum.
This blood vessel, which in the adult is about the size of the fifth finger in diameter, supplies the distal duodenum, the small intestine, and the ascending and transverse colon. Early branches off of the SMA are the pancreaticoduodenal artery (which can serve as a major collateral network between the SMA and celiac axis in occlusive lesions that develop slowly) and the middle colic artery. Since the blood vessel changes caliber with the takeoff of these early branches, most clinically apparent emboli lodge near the origin of the middle colic, leading to ischemia from the proximal jejunum to the splenic flexure (Fig. 1). In 10% to 20% of the population, the right hepatic artery has a replaced origin from the SMA. The SMA terminates as the ileocolic artery. While acute occlusion of the celiac or the interior mesenteric arteries is usually well tolerated, acute SMA occlusion produces widespread infarction throughout the small bowel and colon.
This blood vessel, which in the adult is about the size of the fifth finger in diameter, supplies the distal duodenum, the small intestine, and the ascending and transverse colon. Early branches off of the SMA are the pancreaticoduodenal artery (which can serve as a major collateral network between the SMA and celiac axis in occlusive lesions that develop slowly) and the middle colic artery. Since the blood vessel changes caliber with the takeoff of these early branches, most clinically apparent emboli lodge near the origin of the middle colic, leading to ischemia from the proximal jejunum to the splenic flexure (Fig. 1). In 10% to 20% of the population, the right hepatic artery has a replaced origin from the SMA. The SMA terminates as the ileocolic artery. While acute occlusion of the celiac or the interior mesenteric arteries is usually well tolerated, acute SMA occlusion produces widespread infarction throughout the small bowel and colon.
With abrupt cessation of SMA inflow, small intestinal ischemia rapidly initiates within the usually well-vascularized bowel mucosa, where epithelial and endothelial cell damage lowers mucosal barrier function, leading to bacterial invasion, susceptibility to degrading enzymes, microcirculatory stasis, edema, and intravascular thrombosis. Bacterial and cellular toxins, inflammatory mediators such as cytokines, and particles from cellular death are released into the portal venous circulation and beyond, and drive systemic effects such as increased capillary permeability with edema formation and shock. Even after restoration of organ perfusion, the endothelial cell dysfunction often persists. The resultant ischemia–reperfusion syndrome drives distant multiple organ insufficiency or failure.
The key factor limiting clinical progress in lowering the high morbidity and mortality in acute mesenteric ischemia remains prompt diagnosis; thus, symptoms and signs need to be emphasized. A key to diagnosis is a high index of clinical suspicion in elderly patients, especially those with risk factors such as cardiac dysrhythmias, recent myocardial infarction, valvular heart disease, congestive heart failure, systemic atherosclerosis, and hypercoagulable states such as malignancy. Patients typically note abrupt onset of constant epigastric or midabdominal pain, followed soon after by defecation (sometimes diarrhea and/or rarely blood per rectum) and vomiting. The surgeon should also inquire about evidence of chronic symptoms such as postprandial cramping pain with “food fear,” change in bowel habits, and weight loss, since the distinction between acute versus acute-on-chronic disease may impact technical approaches to bowel revascularization. Finally, in the case of SMA embolization, approximately 25% of patients reveal a history of prior embolic events (brain, leg, etc.).
Physical findings in acute mesenteric thromboembolism can be subtle and nonspecific. The abdomen may be distended with diminished intestinal peristaltic sounds. Classically, abdominal tenderness is less than expected from the patient’s complaints of severe pain. Document a thorough pulse examination to aid in subsequent bypass graft inflow artery selection. Bloody fluid may be retrieved from a nasogastric tube and on rectal examination. Later in the clinical course, as the bowel progresses to infarction, the patient will appear toxic, with shock and peritonitis.
Laboratory tests also yield little sensitivity and specificity. Notable findings may include a leukocytosis with a predominance of immature white blood cells and a metabolic acidosis. D-dimer has been noted to have a high sensitivity for SMA occlusion, but the specificity of this test is low.
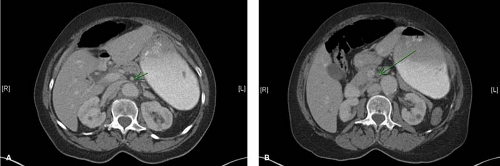
Plain roentgenograms are usually normal but occasionally demonstrate thickened intestinal walls, and in late stages gas in the intestinal wall or portal vein, or peritoneum. While emerging as a useful modality in chronic mesenteric occlusive disease, duplex is of limited benefit in the acute setting due to dilated, air-filled loops of bowel. In cases of diagnostic uncertainty and a clinically stable patient, thin-cut computed tomography (CT) scan with intravenous contrast screens for other pathologies. Relevant acute findings on CT scan include whether the contrast-filled SMA can be identified out to its peripheral branches and bowel abnormalities such as segmental bowel wall thickening. The diagnosis of mesenteric venous obstruction (which is managed primarily with anticoagulation and nutritional support unless it has progressed to significant bowel complications) is made via CT scan—a “halo” or “target” sign can be identified in the superior mesenteric vein. Finally, magnetic resonance angiography can provide anatomic proximal mesenteric vasculature anatomy, though availability and timing limit this modality’s utility in most circumstances.
Visceral biplanar digital subtraction arteriography best defines mesenteric anatomic occlusive lesions. However, angiography is not mandatory, and can result in revascularization delays that adversely affect outcome. In addition to anterior posterior views, a lateral view starting at the first lumbar artery can demonstrate the characteristic appearance of an embolus with a meniscus sign. Nonocclusive mesenteric ischemia (resulting from a depressed cardiac output and oxygen delivery) findings on angiogram include narrowing at the origin of branches of the SMA, spasm of arcade
vessels, and impaired filling of intramural vessels. This syndrome is managed nonoperatively by intra-arterial instillation of dilating compounds (papaverine bolus dose of 60 mg directly into the SMA, followed by a constant infusion of 30 to 60 mg/h until the next angiogram) and physiologic maximization of oxygen delivery.
vessels, and impaired filling of intramural vessels. This syndrome is managed nonoperatively by intra-arterial instillation of dilating compounds (papaverine bolus dose of 60 mg directly into the SMA, followed by a constant infusion of 30 to 60 mg/h until the next angiogram) and physiologic maximization of oxygen delivery.
In patients where there has been a clear intra-abdominal catastrophe (which is often the case with acute mesenteric ischemic), extensive laboratory and radiographic testing unnecessarily delays bowel revascularization, leading to increased bowel loss and higher rates of patient mortality. Resuscitation must proceed expeditiously as the patient is prepared for emergency laparotomy. Surgeons may reasonably immediately move to operative exploration in patients presenting with classical history and physical findings with minimal laboratory testing and no preoperative imaging. Terse preoperative frank yet sensitive discussions with the patient and family can reinforce the life-threatening nature of acute mesenteric ischemia, and this may also serve as a time to introduce the concept of “inoperable” circumstances such as massive bowel infarction. Determination of ischemic versus infarcted bowel remains difficult with currently available clinical and imaging tools, so exploration is most often necessary for assessment. With less dramatic clinical presentations, symptomatic acute mesenteric embolus or thrombosis diagnosed by way of the algorithms outlined above also standardly warrants emergency exploration and bowel revascularization.
The surgeon’s focus on timely clinical diagnosis, immediate laparotomy, and urgent bowel reperfusion via surgical interventions builds from the report of Klass from almost 60 years ago. Patients suffering acute SMA obstruction require multiple parallel interventions in preparation for the operating room. Yet none of these should stand as a rate-limiting step toward skin incision, since these patients can deteriorate rapidly and every minute of delay jeopardizes outcome. Upon diagnosis of acute mesenteric ischemia, the patient is systemically heparinized to minimize clot propagation. Activated clotting times throughout the operative period ensures adequate systemic anticoagulation. Empiric broad spectrum antibiotics are commonly employed since many of these patients have early sepsis, and they additionally serve as some surgical wound prophylaxis (third-generation cephalosporin such as ceftriaxone and metronidazole and in the penicillin-allergic patient a fluoroquinolone and metronidazole). Patients who are health care experienced (recent hospital/nursing home stay, regular visits to hemodialysis centers, etc.) should have their coverage tailored based on local antibiograms. As many of these patients are hypovolemic and have electrolyte abnormalities, fluid volume resuscitation is almost always indicated. Fluid infusion should be targeted to clinical endpoints such as urinary output, skin turgor, and capillary refill; electrolyte abnormalities (especially hypokalemia, hypomagnesemia, and hypophosphatemia) should be corrected as resuscitation proceeds. There is ongoing debate over the optimal type of fluid to use for resuscitation. While the evidence does not support one type of fluid over another, it is best to keep in mind that no matter what type of fluid is used, the clinical response to resuscitation should be closely monitored to prevent both underresuscitation (which can lead to organ failure) and overresuscitation (which can stress the heart and lungs and precipitate myocardial ischemia). All available personnel should work in parallel to place hemodynamic monitors such as arterial lines, secure venous access, a Foley catheter, and a nasogastric tube. Simultaneously, the status of lower extremity pulses is again documented since this patient subset often holds risk factors for lower extremity occlusive disease and may have embolized to the lower extremity arteries.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree