I. STOMACH
The stomach receives food from the esophagus and has four functions: (1) it acts as a reservoir that permits eating reasonably large quantities of food at intervals of several hours; (2) food contained in the stomach is mixed, and delivered into the duodenum in amounts regulated by its chemical nature and texture; (3) the first stages of protein and carbohydrate digestion are carried out in the stomach; and (4) a few substances are absorbed across the gastric mucosa.
The anatomy of the stomach in Figures 23–1, 23–2, and 23–3 illustrates the structure supporting the functions.
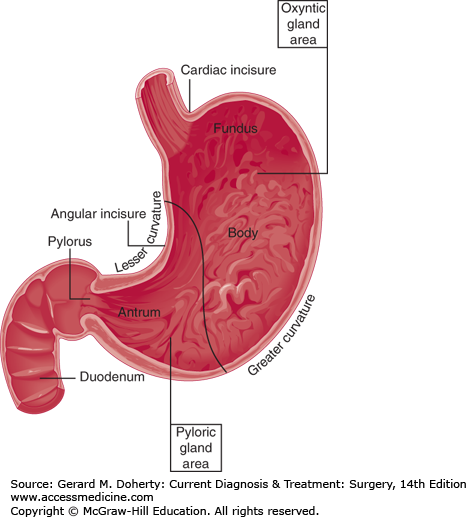
Figure 23–1.
Names of the parts of the stomach. The line drawn from the lesser to the greater curvature depicts the approximate boundary between the oxyntic gland area and the pyloric gland area. No prominent landmark exists to distinguish between antrum and body (corpus). The fundus is the portion craniad to the esophagogastric junction.
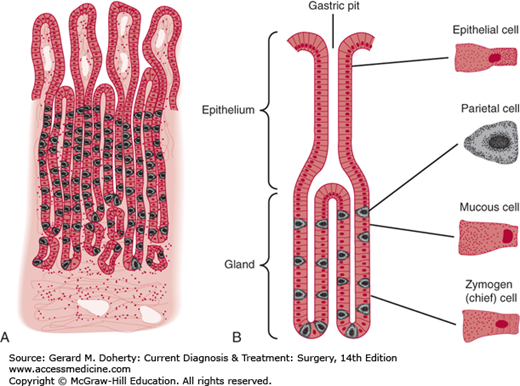
Figure 23–2.
Histologic features of the mucosa in the oxyntic gland area. Each gastric pit drains three to seven tubular gastric glands. A: The neck of the gland contains many mucous cells. Oxyntic (parietal) cells are most numerous in the mid-portion of the glands; peptic (chief) cells predominate in the basal portion. B: Drawing from photomicrograph of the gastric mucosa.
The cardia is located at the gastroesophageal junction. The fundus is the portion of the stomach that lies cephalad to the gastroesophageal junction. The corpus is the capacious central part; division of the corpus from the pyloric antrum is marked approximately by the angular incisure, a crease on the lesser curvature just proximal to the “crow’s-foot” terminations of the nerves of Latarjet (Figure 23–3). The pylorus is the boundary between the stomach and the duodenum.
The cardiac gland area is the small segment located at the gastroesophageal junction. Histologically, it contains principally mucus-secreting cells, though a few parietal cells are sometimes present. The oxyntic gland area is the portion containing parietal (oxyntic) cells and chief cells (Figure 23–2). The boundary between this region and the adjacent pyloric gland area is reasonably sharp, since the zone of transition spans a segment of only 1–1.5 cm. The pyloric gland area constitutes the distal 30% of the stomach and contains the G cells that manufacture gastrin. Mucous cells are common in the oxyntic and pyloric gland areas.
As in the rest of the gastrointestinal tract, the muscular wall of the stomach is composed of an outer longitudinal and an inner circular layer. An additional incomplete inner layer of obliquely situated fibers is most prominent near the lesser curvature but is of less substance than the other two layers.
The blood supply of the stomach and duodenum is illustrated in Figure 23–3. The left gastric artery supplies the lesser curvature and connects with the right gastric artery, a branch of the common hepatic artery. In 60% of persons, a posterior gastric artery arises off the middle third of the splenic artery and terminates in branches on the posterior surface of the body and the fundus. The greater curvature is supplied by the right gastroepiploic artery (a branch of the gastroduodenal artery) and the left gastroepiploic artery (a branch of the splenic artery). The mid-portion of the greater curvature corresponds to a point at which the gastric branches of this vascular arcade change direction. The fundus of the stomach along the greater curvature is supplied by the vasa brevia, branches of the splenic and left gastroepiploic arteries.
The blood supply to the duodenum is from the superior and inferior pancreaticoduodenal arteries, which are branches of the gastroduodenal artery and the superior mesenteric artery, respectively. The stomach contains a rich submucosal vascular plexus. Venous blood from the stomach drains into the coronary, gastroepiploic, and splenic veins before entering the portal vein. The lymphatic drainage of the stomach, which largely parallels the arteries, partially determines the direction of spread of gastric neoplasms.
The parasympathetic nerves to the stomach are shown in Figure 23–3. As a rule, two major vagal trunks pass through the esophageal hiatus in close approximation to the esophageal muscle. The nerves are originally located to the right and left of the esophagus and stomach during embryonic development. When the foregut rotates, the lesser curvature turns to the right and the greater curvature to the left, and corresponding shifts in location of the vagal trunks follow. Hence, the right vagus supplies the posterior and the left the anterior gastric surface. About 90% of the vagal fibers are sensory afferent; the remaining 10% are efferent.
In the region of the gastroesophageal junction, each trunk bifurcates. The anterior trunk sends to the liver a division that travels in the lesser omentum. The bifurcation of the posterior trunk gives rise to fibers that enter the celiac plexus and supply the parasympathetic innervation to the remainder of the gastrointestinal tract as far as the mid-transverse colon. Both trunks, after giving rise to their extragastric divisions, send some fibers directly onto the surface of the stomach and others along the lesser curvature (anterior and posterior nerves of Latarjet) to supply the distal part of the organ. As shown in Figure 23–3, a variable number of vagal fibers ascend with the left gastric artery after having passed through the celiac plexus.
The preganglionic motor fibers of the vagal trunks synapse with ganglion cells in the Auerbach plexus (plexus myentericus) between the longitudinal and circular muscle layers. Postganglionic cholinergic fibers are distributed to the cells of the smooth muscle layers and the mucosa.
The adrenergic innervation to the stomach consists of postganglionic fibers that pass along the arterial vessels from the celiac plexus.
Storage, mixing, trituration, and regulated emptying are accomplished by the muscular apparatus of the stomach. Peristaltic waves originate in the body and pass toward the pylorus. The thickness of the smooth muscle increases in the antrum and corresponds to the stronger contractions that can be measured in the distal stomach. The pylorus behaves as a sphincter, though it normally allows a little to-and-fro movement of chyme across the junction.
An electrical pacemaker situated in the fundal musculature near the greater curvature gives rise to regular (3/min) electrical impulses (pacesetter potential, basic electrical rhythm) that pass toward the pylorus in the outer longitudinal layer. Every impulse is not always followed by a peristaltic muscular contraction, but the impulses determine the maximal peristaltic rate. The frequency of peristalsis is governed by a variety of stimuli mentioned below. Each contraction follows sequential depolarization of the underlying circular muscle resulting from arrival of the pacesetter potential.
Peristaltic contractions are more forceful in the antrum than the body and travel faster as they progress distally. Gastric chyme is forced into the funnel-shaped antral chamber by peristalsis; the volume of contents delivered into the duodenum by each peristaltic wave depends on the strength of the advancing wave and the extent to which the pylorus closes. Most of the gastric contents that are pushed into the antral funnel are propelled backward as the pylorus closes and pressure within the antral lumen rises. Five to 15 mL enter the duodenum with each gastric peristaltic wave.
The volume of the empty gastric lumen is only 50 mL. By a process called receptive relaxation, the stomach can accommodate about 1000 mL before intraluminal pressure begins to rise. Receptive relaxation is an active process mediated by vagal reflexes and abolished by vagotomy. Peristalsis is initiated by the stimulus of distention after eating. Various other factors have positive or negative influences on the rate and strength of contractions and the rate of gastric emptying. Vagal reflexes from the stomach have a facilitating influence on peristalsis. The texture and volume of the meal both play a role in the regulation of emptying; small particles are emptied more rapidly than large ones, which the organ attempts to reduce in size (trituration). The osmolality of gastric chyme and its chemical makeup are monitored by duodenal receptors. If osmolality is greater than 200 mosm/L, a long vagal reflex (the enterogastric reflex) is activated, delaying emptying. Gastrin causes delay in emptying. Gastrin is the only circulating gastrointestinal hormone to have a physiologic effect on emptying.
The output of gastric juice in a fasting subject varies between 500 and 1500 mL/d. After each meal, about 1000 mL are secreted by the stomach.
The components of gastric juice are as follows.
Mucus is a heterogeneous mixture of glycoproteins manufactured in the mucous cells of the oxyntic and pyloric gland areas. Mucus provides a weak barrier to the diffusion of H+ and probably protects the mucosa. It also acts as a lubricant and impedes diffusion of pepsin.
Pepsinogens are synthesized in the chief cells of the oxyntic gland area (and to a lesser extent in the pyloric area) and are stored as visible granules. Cholinergic stimuli, either vagal or intramural, are the most potent pepsigogues, though gastrin and secretin are also effective. The precursor zymogen is activated when pH falls below 5.00, a process that entails severance of a polypeptide fragment from the larger molecule. Pepsin cleaves peptide bonds, especially those containing phenylalanine, tyrosine, or leucine. Its optimal pH is about 2.00. Pepsin activity is abolished at pH greater than 5.00, and the molecule is irreversibly denatured at pH greater than 8.00.
Intrinsic factor, a mucoprotein secreted by the parietal cells, binds with vitamin B12 of dietary origin and greatly enhances absorption of the vitamin. Absorption occurs by an active process in the terminal ileum.
Intrinsic factor secretion is enhanced by stimuli that evoke H+ output from parietal cells. Pernicious anemia is characterized by atrophy of the parietal cell mucosa, deficiency in intrinsic factor, and anemia. Subclinical deficiencies in vitamin B12 have been described after operations that reduce gastric acid secretion, and abnormal Schilling tests in these patients can be corrected by the administration of intrinsic factor. Total gastrectomy creates a dependence on parenteral administration of vitamin B12.
The unique characteristic of gastric secretion is its high concentration of hydrochloric acid, a product of the parietal cells. As the concentration of H+ rises during secretion, that of Na+ drops in a reciprocal fashion. K+ remains relatively constant at 5–10 mEq/L. Chloride concentration remains near 150 mEq/L, and gastric juice maintains its isotonicity at varying secretory rates.
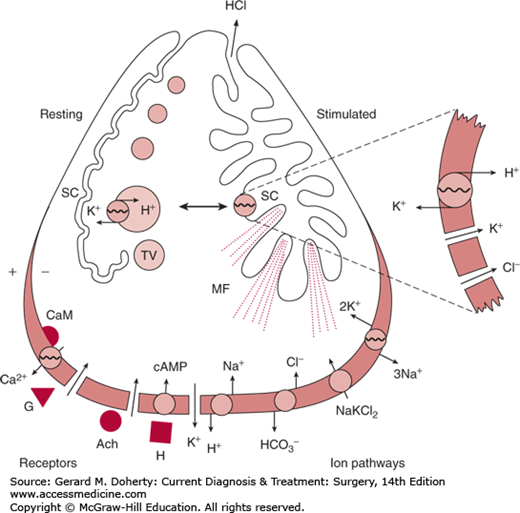
Many of the key events in acid secretion by gastric parietal cells are illustrated in Figure 23–4. The onset of secretion is accompanied by striking morphologic changes in the apical membranes. Resting parietal cells are characterized by an infolding of the apical membrane, called the secretory canaliculus, which is lined by short microvilli. Multiple membrane-bound tubulovesicles and mitochondria are present in the cytoplasm. With stimulation, the secretory canaliculus expands, the microvilli become long and narrow and filled with microfilaments, and the cytoplasmic tubulovesicles disappear. The proton pump mechanism for acid secretion is located in the tubulovesicles in the resting state and in the secretory canaliculus in the stimulated state.
Figure 23–4.
Diagram of a parietal cell, showing the receptor systems and ion pathways in the basal lateral membrane and the apical membrane transition from a resting to a stimulated state. Ach, acetylcholine; CaM, calmodulin; G, gastrin; H, histamine; MF, microfilaments; SC, secretory canaliculus; TV, tubulovesicles. (Redrawn, with permission, from Malinowska DH, Sachs G: Cellular mechanisms of acid secretion. Clin Gastroenterol. 1984;13(2):309–326.)
The basal lateral membrane contains the receptors for secretory stimulants and transfers HCO3− out of the cell to balance the H+ output at the apical membrane. Active uptake of Cl− and K+ conduction also occur at the basal lateral membrane. Separate membrane-bound receptors exist for histamine (H2 receptor), gastrin, and acetylcholine. The intracellular second messengers are cyclic adenosine monophosphate (cAMP) for histamine and Ca2+ for gastrin and acetylcholine.
Acid secretion at the apical membrane is accomplished by a membrane-bound H+/K+-ATPase (the proton pump); H+ is secreted into the lumen in exchange for K+.
The healthy mucosa of the stomach and duodenum is provided with mechanisms that allow it to withstand the potentially injurious effects of high concentrations of luminal acid. Disruption of these mechanisms may contribute to acute or chronic ulceration.
The surface of the gastric mucosa is coated with mucus and secretes HCO3− in addition to H+. Protected by the blanket of mucus, the surface pH is much higher than the luminal pH. HCO3− secretion is stimulated by cAMP, prostaglandins, cholinomimetics, glucagon, cholecystokinin, and by as yet unidentified paracrine hormones. Inhibitors of HCO3− secretion include nonsteroidal anti-inflammatory agents, alpha-adrenergic agonists, bile acids, ethanol, and acetazolamide. Increases in luminal H+ result in increased HCO3− secretion, probably mediated by tissue prostaglandins.
Gastric mucus is a gel composed of high-molecular-weight glycoproteins and 95% water. Since it forms an unstirred layer, it helps the underlying mucosa to maintain a higher pH than that of gastric juice, and it also acts as a barrier to the diffusion of pepsin. At the surface of the layer of mucus, peptic digestion continuously degrades mucus, while below it is continuously being replenished by mucous cells. Gastric acid is thought to enter the lumen through thin spots in the mucus overlying the gastric glands. Secretion of mucus is stimulated by luminal acid and perhaps by cholinergic stimuli. The layer of mucus is damaged by exposure to nonsteroidal anti-inflammatory agents and is enhanced by topical prostaglandin E2.
Mucosal defects produced by mechanical or chemical trauma are rapidly repaired by adjacent normal cells that spread to cover the defect, a process that can be enhanced experimentally by adding HCO3− to the nutrient side of the mucosa.
The duodenal mucosa possesses defenses similar to those in the stomach: the ability to secrete HCO3− and mucus and rapid repair of mucosal injuries.
The regulation of acid secretion can best be described by considering separately those factors that enhance gastric acid production and those that depress it. The interaction of these forces is what determines the levels of secretion observed during fasting and after meals.
Acid production is usually described as the result of three phases that are excited simultaneously after a meal. The separation into phases is of value principally for descriptive purposes.
Stimuli that act upon the brain lead to increased vagal efferent activity and acid secretion. The sight, smell, taste, or even thought of appetizing food may elicit this response. The effect is vagally mediated and is abolished by vagotomy. The vagal stimuli have a direct effect on the parietal cells to increase acid output.
Food in the stomach (principally protein hydrolysates and hydrophobic amino acids) stimulates gastrin release from the antrum. Gastric distention has a similar but less intense effect.
The presence of food in the stomach excites long vagal reflexes, impulses that pass to the central nervous system via vagal afferents and return to stimulate the parietal cells.
A third aspect of the gastric phase involves the sensitizing effect of distention of the parietal cell area to gastrin that is probably mediated through local intramural cholinergic reflexes.
The role of the intestinal phase in the stimulation of gastric secretion has been incompletely investigated. Various experiments have shown that the presence of food in the small bowel releases a humoral factor, named entero-oxyntin, that evokes acid secretion from the stomach.
Without systems to limit secretion, unchecked acid production could become a serious clinical problem. Examples can be found (Billroth II gastrectomy with retained antrum) where acid production rose after surgical procedures that interfered with these inhibitory mechanisms.
pH below 2.50 in the antrum inhibits the release of gastrin regardless of the stimulus. When the pH reaches 1.20, gastrin release is almost completely blocked. If the normal relationship of parietal cell mucosa to antral mucosa is changed so that acid does not flow past the site of gastrin production, serum gastrin may increase to high levels, with marked acid stimulation. Somatostatin in gastric antral cells serves a physiologic role as an inhibitor of gastrin release (a paracrine function).
The intestine participates in controlling acid secretion by liberating hormones that inhibit both the release of gastrin and its effects on the parietal cells. Secretin blocks acid secretion under experimental conditions but not as a physiologic action. Fat in the intestine is the most potent method of inhibition, affecting gastrin release and acid secretion.
Ingested food is mixed with salivary amylase before it reaches the stomach. The mechanisms stimulating gastric secretion are activated. Serum gastrin levels increase from a mean fasting concentration of about 50 pg/mL to 200 pg/mL, the peak occurring about 30 minutes after the meal. Food in the lumen of the stomach is exposed to high concentrations of acid and pepsin at the mucosal surface. Food settles in layers determined by sequence of arrival, but fat tends to float to the top. The greatest mixing occurs in the antrum. Antral contents therefore become more uniformly acidic than those in the body of the organ, where the central portion of the meal tends to remain alkaline for a considerable time, allowing continued activity of the amylase.
Peptic digestion of protein in the stomach is only about 5%–10% complete. Carbohydrate digestion may reach 30%–40%. A lipase originating from the tongue initiates the first stages of lipolysis in the stomach.
The gastric contents are delivered to the duodenum at a rate determined by the volume and texture of the meal, its osmolality and acidity, and its content of fat. A meal of lean meat, potatoes, and vegetables leaves the stomach within 3 hours. A meal with a very high fat content may remain in the stomach for 6–12 hours.
Peptic ulcers result from the corrosive action of acid gastric juice on a vulnerable epithelium. Depending on circumstances, they may occur in the esophagus, the duodenum, the stomach itself, the jejunum after surgical construction of a gastrojejunostomy, or the ileum in relation to ectopic gastric mucosa in Meckel diverticulum. When the term peptic ulcer was first used, it was thought that the most important factor was the peptic activity in gastric juice. Since then, evidence has implicated acid as the chief injurious agent; in fact, it is axiomatic that if gastric juice contains no acid, a (benign) peptic ulcer cannot be present. Appreciation of the role of acid has led to the emphasis on therapy with antacids and H2 blocking agents for the medical therapy of ulcers and to operations that reduce acid secretion as the major surgical approach. In the case of duodenal and gastric ulcers, Helicobacter pylori must colonize and weaken the mucosa before acid is able to do the damage, and therapy directed against this organism has a more definitive effect on the disease.
It has been estimated that about 2% of the adult population in the United States suffers from active peptic ulcer disease, and about 10% of the population will have the disease during their lifetime. Men are affected three times as often as women. Duodenal ulcers are ten times more common than gastric ulcers in young patients, but in the older age groups the frequency is about equal. Probably as a result of a declining prevalence of H pylori infection, the incidence has declined to less than half what it was 30 years ago.
In general terms, the ulcerative process can lead to four types of disability: (1) Pain is the most common. (2) Bleeding may occur as a result of erosion of submucosal or extraintestinal vessels as the ulcer becomes deeper. (3) Penetration of the ulcer through all layers of the affected gut results in perforation if other viscera do not seal the ulcer. (4) Obstruction may result from inflammatory swelling and scarring and is most likely to occur with ulcers located at the pylorus or gastroesophageal junction, where the lumen is narrowest.
The clinical features and prognosis of duodenal ulcer and gastric ulcer are sufficiently different to be dealt with separately here.
ESSENTIALS OF DIAGNOSIS
Epigastric pain often relieved by food or antacids
Epigastric tenderness
Normal or increased gastric acid secretion
Signs of ulcer disease on upper gastrointestinal x-rays or endoscopy
Evidence of H pylori infection
Duodenal ulcers may occur in any age group but are most common in the young and middle-aged (20–45 years). They appear in men more often than women. About 95% of duodenal ulcers are situated within 2 cm of the pylorus, in the duodenal bulb.
Considerable evidence implicates H pylori as the principal cause of duodenal ulcer disease. This microaerophilic gram-negative curved bacillus can be found colonizing patches of gastric metaplasia within the duodenum in 90% of patients with this disease. The bacilli remain on the surface of the mucosa rather than invading it. They are thought to render the duodenum more vulnerable to the injurious effects of acid and pepsin by releasing urease or other toxins.
The epidemiology of peptic ulcer disease reflects the prevalence of H pylori infection in different populations. In areas of the world where peptic ulcer is uncommon (eg, rural Africa), human infection is rare. Duodenal ulcer disease has emerged as a major clinical entity in Western society only since the latter part of the 19th century. The incidence reached a peak about 35 years ago and then declined to reach a lower plateau a few years ago. These changes are thought to be explained by variations in H pylori infection resulting from public health factors. Within countries like the United States, the distribution of H pylori is explainable by a fecal-oral theory of transmission. The prevalence of infection is higher among lower socioeconomic groups. Interestingly, only a minority of infected persons develop ulcers. H pylori also has an important role in the etiology of gastric ulcer, gastric cancer, and gastritis. The 10% of duodenal ulcers that are not associated with helicobacter infection are caused by nonsteroidal anti-inflammatory drugs and other agents.
Gastric acid secretion is characteristically higher than normal in patients with duodenal ulcer compared with normal subjects, but only one-sixth of the duodenal ulcer population have secretory levels that exceed the normal range (ie, acid secretion in normal subjects and those with duodenal ulcer overlap considerably), so the disease cannot be explained simply as a manifestation of increased acid production. Whether acid secretion increases in response to helicobacter infection is doubted. One possibility is that the patches of metaplastic gastric epithelium in the duodenum on which helicobacter take up residence result from the action of acid. Then the colonized patches undergo ulceration.
Chronic liver disease, chronic lung disease, and chronic pancreatitis have all been implicated as increasing the possibility of duodenal ulceration.
Pain, the presenting symptom in most patients, is usually located in the epigastrium and is variably described as aching, burning, or gnawing. Radiologic survey studies indicate, however, that some patients with active duodenal ulcer have no gastrointestinal complaints.
The daily cycle of the pain is often characteristic. The patient usually has no pain in the morning until an hour or more after breakfast. The pain is relieved by the noon meal, only to recur in the later afternoon. Pain may appear again in the evening, and in about half of cases, it arouses the patient during the night. Food, milk, or antacid preparations give temporary relief.
When the ulcer penetrates the head of the pancreas posteriorly, back pain is noted; concomitantly, the cyclic pattern of pain may change to a more steady discomfort, with less relief from food and antacids.
Varying degrees of nausea and vomiting are common. Vomiting may be a major feature even in the absence of obstruction.
The abdominal examination may reveal localized epigastric tenderness to the right of the midline, but in many instances no tenderness can be elicited.
Gastroduodenoscopy is useful in evaluating patients with an uncertain diagnosis, those with bleeding from the upper intestine, and those who have obstruction of the gastroduodenal segment and for assessing response to therapy.
A gastric analysis may be indicated in certain cases. The standard gastric analysis consists of the following: (a) Measurement of acid production by the unstimulated stomach under basal fasting conditions; the result is expressed as H+ secretion in mEq/h and is termed the basal acid output (BAO). (b) Measurement of acid production during stimulation by histamine or pentagastrin given in a dose maximal for this effect. The result is expressed as H+ secretion in mEq/h and is termed the maximal acid output (MAO).
Interpretation of the results is outlined in Table 23–1.
| Mean Acid Output (meq/h) | |||
|---|---|---|---|
| Sex | Normal | Duodenal Ulcer | |
| Basal | Male | 2.5 | 5.5 |
| Female | 1.5 | 3 | |
| Maximal (pentagastrin) | Male | 30 | 40 |
| Female | 20 | 30 | |
Depending on the laboratory, normal basal gastrin levels average 50–100 pg/mL, and levels over 200 pg/mL can almost always be considered high.
Gastrin concentrations may rise in hyposecretory and hypersecretory states. In the former conditions (eg, atrophic gastritis, pernicious anemia, acid-suppressant medications), the cause is higher antral pH with loss of antral inhibition for gastrin release. More important clinically is elevated gastrin levels with concomitant hypersecretion, where the high gastrin level is responsible for the increased acid and resulting peptic ulceration. The best-defined clinical condition in this category is Zollinger–Ellison syndrome (gastrinoma). Antrum attached to the duodenum, but out of continuity with the gastric alimentary flow after gastrectomy (retained antrum), is another cause of elevated gastrin driving excess gastric acid secretion.
A fasting serum gastrin determination should be obtained in patients with peptic ulcer disease that is unusually severe or refractory to therapy.
On an upper gastrointestinal series or CT scan with gastrointestinal contrast, the changes induced by duodenal ulcer consist of duodenal deformities and an ulcer niche. Inflammatory swelling and scarring may lead to distortion of the duodenal bulb, eccentricity of the pyloric channel, or pseudodiverticulum formation. The ulcer itself may be seen either in profile or, more commonly, en face.
The most common diseases simulating peptic ulcer are (1) chronic cholecystitis, in which cholecystograms show either nonfunctioning of the gallbladder or stones in a functioning gallbladder; (2) acute pancreatitis, in which the serum amylase is elevated; (3) chronic pancreatitis, in which endoscopic retrograde cholangiopancreatography (ERCP) shows an abnormal pancreatic duct; (4) functional indigestion, in which x-rays are normal; and (5) reflux esophagitis.
The common complications of duodenal ulcer are hemorrhage, perforation, and duodenal obstruction. Each of these is discussed in a separate section. Less common complications are pancreatitis and biliary obstruction.
Prevention of ulcer disease entails avoidance of H pylori infection.
Acute duodenal ulcer can be controlled by suppressing acid secretion in most patients, but the long-term course of the disease (ie, frequency of relapses and of complications) is unaffected unless H pylori infection is eradicated. Surgical therapy is recommended principally for the treatment of complications: bleeding, perforation, or obstruction.
The goals of medical therapy are: (1) to heal the ulcer and (2) to cure the disease. Treatment in the first category is aimed at decreasing acid secretion or neutralizing acid. The principal drugs consist of H2 receptor antagonists (eg, cimetidine, ranitidine) and proton pump blockers (eg, omeprazole, pantoprazole).
After the ulcer has healed, discontinuation of therapy results in an 80% recurrence rate within 1 year, which may be avoided by chronic nighttime administration of a single dose of acid suppressive agent. A better approach is to treat the H pylori infection along with the ulcer, since eradication of H pylori diminishes recurrent ulceration unless the infection recurs–an uncommon event. The following combination is an effective regimen: lansoprazole, 30 mg twice daily for 14 days; amoxicillin, 1 g twice daily for 14 days; and clarithromycin, 500 mg twice daily for 14 days.
If medical treatment has been optimal, a persistent ulcer may be judged intractable, and surgical treatment is indicated. This is now uncommon.
The surgical procedures that can cure peptic ulcer are aimed at reduction of gastric acid secretion. Excision of the ulcer itself is not sufficient for either duodenal or gastric ulcer; recurrence is nearly inevitable with such procedures.
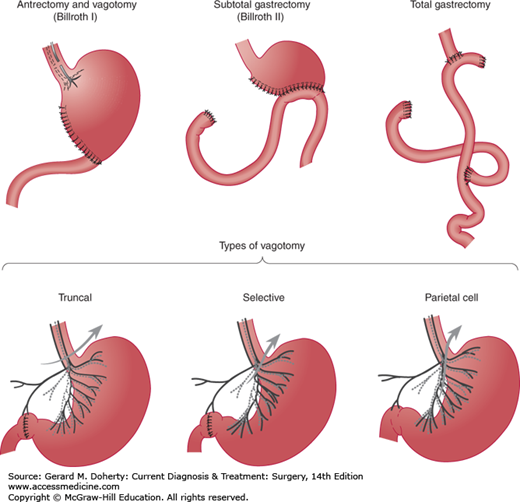
The surgical methods of treating duodenal ulcer are vagotomy (several varieties) and antrectomy plus vagotomy. All of these procedures can be performed laparoscopically. With rare exceptions, one of the vagotomy operations is sufficient (Figure 23–5).
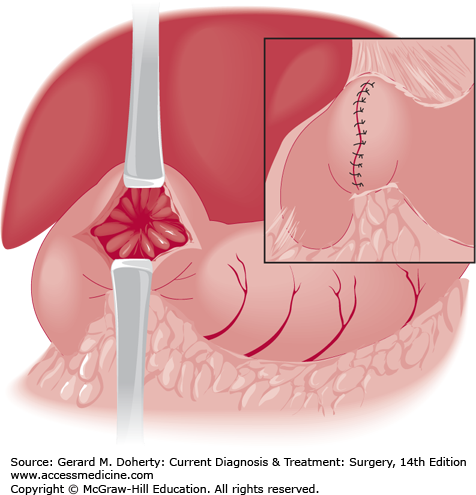
Truncal vagotomy consists of resection of a 1- or 2-cm segment of each vagal trunk as it enters the abdomen on the distal esophagus. The resulting vagal denervation of the gastric musculature produces delayed emptying of the stomach in many patients unless a drainage procedure is performed. The method of drainage most often selected is pyloroplasty (Heineke–Mikulicz procedure, Figure 23–6); gastrojejunostomy is used less often. Neither procedure gives a superior functional result, and pyloroplasty is less time consuming.
Vagal denervation of just the parietal cell area of the stomach is called parietal cell vagotomy or proximal gastric vagotomy. The technique spares the main nerves of Latarjet (Figures 23–3 and 23–5) but divides all vagal branches that terminate on the proximal two-thirds of the stomach. Since antral innervation is preserved, gastric emptying is relatively normal, and a drainage procedure is unnecessary. Nevertheless, parietal cell vagotomy plus pyloroplasty gives better results (ie, fewer recurrent ulcers) than parietal cell vagotomy alone. Parietal cell vagotomy appears to have about the same effectiveness as truncal or selective vagotomy for curing the ulcer disease, but dumping and diarrhea are much less frequent.
The vagotomy procedures have the advantages of technical simplicity and preservation of the entire gastric reservoir capacity. The principal disadvantage is recurrent ulceration in about 10% of patients. The recurrence rate after parietal cell vagotomy is about twice as high in patients with prepyloric ulcer, and most surgeons use a different operation for an ulcer in this location.
This operation entails a distal gastrectomy of 50% of the stomach, with the line of gastric transection carried high on the lesser curvature to conform with the boundary of the gastrin-producing mucosa.
The terms antrectomy and hemigastrectomy are loosely synonymous. The proximal remnant may be reanastomosed to the duodenum (Billroth I resection) or to the side of the proximal jejunum (Billroth II resection). The Billroth I technique is most popular, but there is no conclusive evidence that the results are superior. When creating a Billroth II (gastrojejunostomy) reconstruction, the surgeon may bring the jejunal loop up to the gastric remnant either anterior to the transverse colon or posteriorly through a hole in the transverse mesocolon. Since either method is satisfactory, an antecolic anastomosis is elected in most cases because it is simpler. Truncal vagotomy is performed as described in the preceding section; antrectomy by itself will not prevent a high recurrence rate. In most instances, the surgeon will be able to remove the ulcerated portion of duodenum in the course of resection.
Vagotomy and antrectomy is associated with a low incidence of marginal ulceration (2%) and a generally good overall outcome, but the risk of complications is higher than after vagotomy without resection.
This operation consists of resection of two-thirds to three-fourths of the distal stomach. After subtotal gastrectomy for duodenal ulcer, a Billroth II reconstruction is preferable. Subtotal gastrectomy is largely of historical interest.
Duodenal stump leakage, gastric retention (poor gastric emptying), and hemorrhage may develop in the immediate postoperative period.
Recurrent ulcers formed in about 10% of duodenal ulcer patients treated by vagotomy and pyloroplasty or parietal cell vagotomy; and in 2%–3% after vagotomy and antrectomy or subtotal gastrectomy without chronic acid suppression. Recurrent ulcers nearly always develop immediately adjacent to the anastomosis on the intestinal side.
The usual complaint is upper abdominal pain, which is often aggravated by eating and improved by antacids. In some patients, the pain is felt more to the left in the epigastrium, and left axillary or shoulder pain is occasionally reported. About a third of patients with stomal ulcer have major gastrointestinal hemorrhage. Free perforation is less common (5%).
Diagnosis and treatment are essentially the same as for the original ulcer.
A deeply eroding ulcer may occasionally produce a fistula between the stomach and colon. Most examples have resulted from recurrent peptic ulcer after an operation that included a gastrojejunal anastomosis.
Severe diarrhea and weight loss are the presenting symptoms in over 90% of cases. Abdominal pain typical of recurrent peptic ulcer often precedes the onset of the diarrhea. Bowel movements number 8–12 or more a day; they are watery and often contain particles of undigested food.
The degree of malnutrition ranges from mild to very severe. Laboratory studies reveal low serum proteins and manifestations of fluid and electrolyte depletion. Appropriate tests may reflect deficiencies in both water-soluble and fat-soluble vitamins.
An upper gastrointestinal series reveals the marginal ulcer in only 50% of patients and the fistula in only 15%. Barium enema unfailingly demonstrates the fistulous tract.
Initial treatment should replenish fluid and electrolyte deficits. The involved colon and ulcerated gastrojejunal segment should be excised and colonic continuity reestablished. Vagotomy, partial gastrectomy, or both are required to treat the ulcer diathesis and prevent another recurrent ulcer. Results are excellent in benign disease. In general, the outlook for patients with a malignant fistula is poor.
Symptoms of the dumping syndrome are noted to some extent by most patients who have an operation that impairs the ability of the stomach to regulate its rate of emptying. Within several months, however, dumping is a clinical problem in only 1%–2% of patients. Symptoms fall into two categories: cardiovascular and gastrointestinal. Shortly after eating, the patient may experience palpitations, sweating, weakness, dyspnea, flushing, nausea, abdominal cramps, belching, vomiting, diarrhea, and, rarely, syncope. The degree of severity varies widely, and not all symptoms are reported by all patients. In severe cases, the patient must lie down for 30–40 minutes until the discomfort passes.
Diet therapy to reduce jejunal osmolality is successful in all but a few cases. The diet should be low in carbohydrate and high in fat and protein content. Sugars and carbohydrates are least well tolerated; some patients are especially sensitive to milk. Meals should be taken dry, with fluids restricted to between meals. This dietary regimen ordinarily suffices, but anticholinergic drugs may be of help in some patients; others have reported improvement with supplemental pectin in the diet, or the use of somatostatin analogs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree