Status Epilepticus
KEY CONCEPTS
![]() Status epilepticus (SE) is a neurologic emergency that is associated with significant morbidity and mortality.
Status epilepticus (SE) is a neurologic emergency that is associated with significant morbidity and mortality.
![]() Generalized convulsive status epilepticus (GCSE) is defined as any recurrent or continuous seizure activity lasting longer than 30 minutes in which the patient does not regain baseline mental status. Any seizure that does not stop within 5 minutes should be treated as impending SE.
Generalized convulsive status epilepticus (GCSE) is defined as any recurrent or continuous seizure activity lasting longer than 30 minutes in which the patient does not regain baseline mental status. Any seizure that does not stop within 5 minutes should be treated as impending SE.
![]() There are two types of SE, GCSE and nonconvulsive status epilepticus (NCSE). GCSE is the most common type.
There are two types of SE, GCSE and nonconvulsive status epilepticus (NCSE). GCSE is the most common type.
![]() Most GCSE develops in patients with no history of epilepsy; however, a patient with preexisting epilepsy may experience GCSE as a result of acute anticonvulsant withdrawal, metabolic disorder, concurrent illness, or progression of neurologic disease.
Most GCSE develops in patients with no history of epilepsy; however, a patient with preexisting epilepsy may experience GCSE as a result of acute anticonvulsant withdrawal, metabolic disorder, concurrent illness, or progression of neurologic disease.
![]() Although the pathophysiology of GCSE is unknown, experimental models have shown that there is a dramatic decrease in γ-aminobutyric acid–mediated inhibitory synaptic transmission and that glutamatergic excitatory synaptic transmission sustains the seizures.
Although the pathophysiology of GCSE is unknown, experimental models have shown that there is a dramatic decrease in γ-aminobutyric acid–mediated inhibitory synaptic transmission and that glutamatergic excitatory synaptic transmission sustains the seizures.
![]() General treatment includes patient stabilization, adequate oxygenation, preservation of cardiorespiratory function, management of systemic complications, and aggressive assessment of underlying causes.
General treatment includes patient stabilization, adequate oxygenation, preservation of cardiorespiratory function, management of systemic complications, and aggressive assessment of underlying causes.
![]() The main purpose of treatment is to prevent or decrease morbidity and mortality of prolonged seizures. Pharmacologic treatment needs to be rapid and aimed at terminating both electrical and clinical seizures. The probability of poorer outcomes increases with increased length of electrographic seizure activity.
The main purpose of treatment is to prevent or decrease morbidity and mortality of prolonged seizures. Pharmacologic treatment needs to be rapid and aimed at terminating both electrical and clinical seizures. The probability of poorer outcomes increases with increased length of electrographic seizure activity.
![]() Lorazepam is the preferred benzodiazepine in treatment of GCSE because of its efficacy and long duration of action in the CNS. Midazolam is the preferred benzodiazepine for intramuscular (IM) administration.
Lorazepam is the preferred benzodiazepine in treatment of GCSE because of its efficacy and long duration of action in the CNS. Midazolam is the preferred benzodiazepine for intramuscular (IM) administration.
![]() Currently, the hydantoins (phenytoin and fosphenytoin) are the long-acting anticonvulsants used most frequently. Either phenytoin or fosphenytoin should be given concurrently with benzodiazepines.
Currently, the hydantoins (phenytoin and fosphenytoin) are the long-acting anticonvulsants used most frequently. Either phenytoin or fosphenytoin should be given concurrently with benzodiazepines.
![]() The maximum rate of infusion for phenytoin and fosphenytoin in adults is 50 mg/min and 150 mg PE/min, respectively.
The maximum rate of infusion for phenytoin and fosphenytoin in adults is 50 mg/min and 150 mg PE/min, respectively.
![]() If GCSE is not controlled by two first-line agents (benzodiazepine plus hydantoin or phenobarbital), the GCSE is considered to be refractory. In these cases, anesthetic doses of midazolam, pentobarbital, or propofol may be used.
If GCSE is not controlled by two first-line agents (benzodiazepine plus hydantoin or phenobarbital), the GCSE is considered to be refractory. In these cases, anesthetic doses of midazolam, pentobarbital, or propofol may be used.
INTRODUCTION
![]() Status epilepticus (SE) is a common neurologic emergency that is associated with brain damage and death.
Status epilepticus (SE) is a common neurologic emergency that is associated with brain damage and death. ![]() The traditional definition defines SE as (a) any seizure lasting longer than 30 minutes whether or not consciousness is impaired or (b) recurrent seizures without an intervening period of consciousness between seizures.1 Clinically, this definition has limited use, as the average seizure is less than 2 minutes; and only 40% of seizures lasting 10 to 29 minutes cease without treatment.2,3 Pharmacoresistance4 and mortality3 significantly increase with prolonged seizure duration.
The traditional definition defines SE as (a) any seizure lasting longer than 30 minutes whether or not consciousness is impaired or (b) recurrent seizures without an intervening period of consciousness between seizures.1 Clinically, this definition has limited use, as the average seizure is less than 2 minutes; and only 40% of seizures lasting 10 to 29 minutes cease without treatment.2,3 Pharmacoresistance4 and mortality3 significantly increase with prolonged seizure duration. ![]() Therefore, aggressive treatment of seizures lasting 5 minutes or more is strongly recommended.
Therefore, aggressive treatment of seizures lasting 5 minutes or more is strongly recommended. ![]() SE can present in several forms (Table 41-1), including generalized convulsive status epilepticus (GCSE) and nonconvulsive status epilepticus (NCSE).1
SE can present in several forms (Table 41-1), including generalized convulsive status epilepticus (GCSE) and nonconvulsive status epilepticus (NCSE).1
TABLE 41-1 International Classification of Status Epilepticus
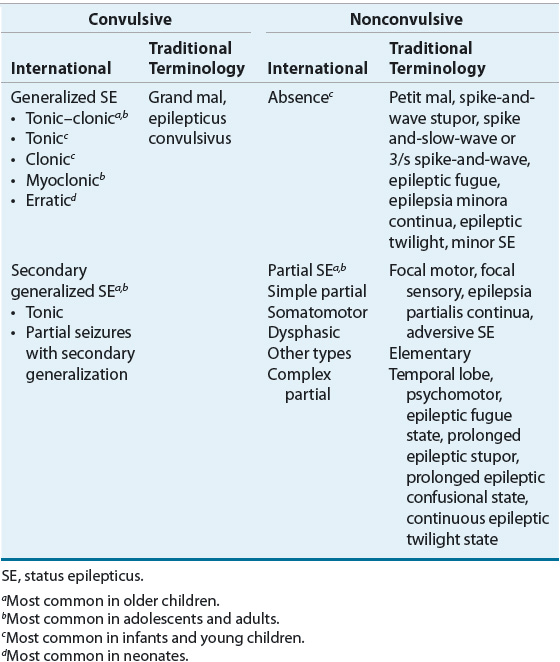
NCSE occurs in 25% of those with SE and is characterized by a fluctuating or continuous “epileptic twilight” state that produces altered consciousness and/or behavior (e.g., lethargy, decreased mental function). An electroencephalogram (EEG) is the most important diagnostic and management tool.5 In most instances, a benzodiazepine and/or valproate remain drugs of choice.5 Although IV hydantoin, levetiracetam, or phenobarbital can be tried in nonresponders, general anesthesia is usually not appropriate.5
This chapter will focus on GCSE, which is the most common and severe form of SE. It is characterized by repeated primary or secondary generalized seizures that involve both hemispheres of the brain and are associated with a persistent postictal state.4
EPIDEMIOLOGY
The worldwide and United States incidence ranges between 1.2 to 5 million and 100,000 to 152,000 cases each year, respectively.4 GCSE has no predilection for gender or socioeconomic status but does occur more frequently in nonwhites across all ages.6 Most GCSE occurs in individuals with no history of epilepsy; however, approximately 5% of adults and 10% to 25% of children with epilepsy will develop GCSE.7 The incidence of GCSE is highest in those younger than 1 year of age and in those older than 60 years of age.
ETIOLOGY
Precipitating events for GCSE vary and generally reflect different populations and referral patterns. ![]() Most episodes in individuals with epilepsy occur because of acute anticonvulsant withdrawal, a metabolic disorder or concurrent illness, or progression of a preexisting neurologic disease. Common etiologies and mortality rates are shown in Table 41-2.6,8 Precipitating events are divided into those with and without neurologic structural lesions or those with a precipitating injury or insult. Cases with structural lesions or those with a specific neurologic insult are associated with a poor prognosis.
Most episodes in individuals with epilepsy occur because of acute anticonvulsant withdrawal, a metabolic disorder or concurrent illness, or progression of a preexisting neurologic disease. Common etiologies and mortality rates are shown in Table 41-2.6,8 Precipitating events are divided into those with and without neurologic structural lesions or those with a precipitating injury or insult. Cases with structural lesions or those with a specific neurologic insult are associated with a poor prognosis.
TABLE 41-2 Etiology and Mortality for Pediatric and Adult Cases of Status Epilepticus
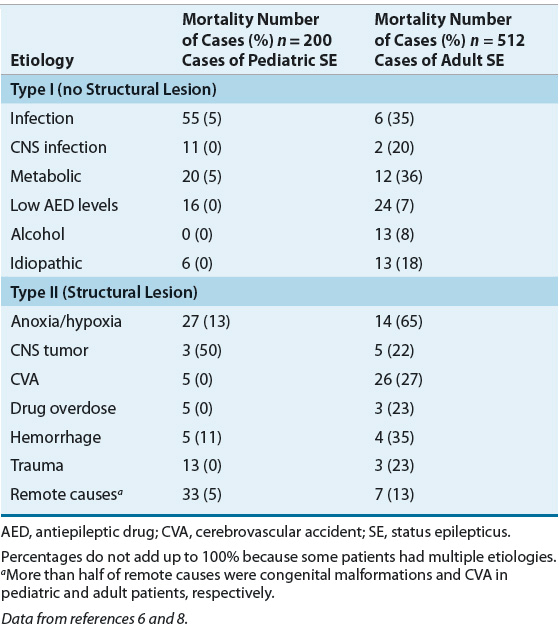
There are major differences in etiologies for pediatric and adult patients (see Table 41-2). During their first few weeks of life, infants who are born to addicted mothers can develop drug withdrawal seizures. Other neonates can develop GCSE because of pyridoxine deficiency, which should resolve within hours following IV pyridoxine (100 mg). Acute encephalopathy and metabolic disorders are the major causes of GCSE in those younger than 1 year of age. In young children, the cause is often a nonspecific illness such as fever and/or a viral illness. The most frequent precipitating events in adults are cerebrovascular disease, rapid anticonvulsant withdrawal, and low anticonvulsant serum concentrations. Cerebrovascular disease is the leading cause in those who have their first seizures after age 60. Prescription, over-the-counter, and recreational drugs should be considered in anyone with new-onset GCSE.
MORBIDITY AND MORTALITY
GCSE is harmful to the brain. While most contend that the GCSE is responsible for the damage, it is unknown if the morbidity results from the underlying etiology or the GCSE. Regardless of the inducing stimulus, neuronal damage in animal models is evident following 30 to 60 minutes of GCSE, and most progress to develop epilepsy following a prolonged seizure. Interestingly, inhibiting the seizure-induced neuronal damage does not prevent the development of epilepsy, suggesting that the seizures themselves may be harmful. It is hard to establish a relationship between GCSE and long-term outcomes because it is difficult to weigh the effects of seizure type, etiology, duration, concurrent physiologic events, and therapy or lack thereof. It has been shown that patients with a history of prolonged febrile seizures who later developed epilepsy share similar histopathologic changes (i.e., hippocampal sclerosis) to those found in animal models of GCSE.9,10 In these cases, the period between the initial GCSE and the first epileptic seizure may be months to decades, suggesting a possible link between GCSE and the development of epilepsy. Importantly, studies of GCSE show that the currently available anticonvulsants do not reproducibly prevent the development of epilepsy following prolonged seizures.9,11
Patients who develop epilepsy following prolonged GCSE are less likely to experience remission of their seizures and may have decreased cognitive and memory function, mental retardation, or neurologic deficits when compared to those who develop epilepsy and subsequently have GCSE.4 Most studies have found that younger children, the elderly, and those with preexisting epilepsy have a higher propensity for sequelae. Unless accompanied by an underlying neurologic abnormality, febrile status epilepticus is less likely to be associated with sequelae.
Estimated mortality in the United States following GCSE ranges between 22,000 and 42,000 individuals per year,8 with rates up to 16% in children,12 20% in adults,4 and 38% in the elderly.6 When compared with other populations, neonates have a higher mortality and more neurologic sequelae.
Table 41-2 summarizes the etiology and corresponding mortality rates for GCSE.6,8 Interestingly, the mortality associated with many etiologies is significantly greater in adults than in children. Unresponsive patients may die from GCSE, but more frequently they die from the acute illness that precipitated the GCSE. For example, patients with serious CNS structural changes (e.g., hemorrhage, stroke) have a poor prognosis, compared to those with no structural lesion.
Outcome is affected by the time between onset of GCSE and the initiation of treatment and the duration of the seizure. Mortality significantly increases with increased seizure duration (e.g., 2.6% for seizures 10 to 29 minutes, 19% for seizures lasting >30 minutes, and 32% for seizures lasting greater than 60 minutes).2,8 Mortality has decreased over the past decade and probably reflects a recognition of the need to initiate sequenced therapy using large doses as soon as possible.
PATHOGENESIS
Seizures occur when the excitatory neurotransmission overcomes inhibitory impulses in one or more brain regions. Most seizures are brief (less than 5 minutes), largely because the brain’s inhibitory mechanisms restore the balance of normal neurotransmission.4 Although it is unknown why the mechanisms that control normal brain homeostasis fail, when seizures occur in close succession or the magnitude of the proconvulsant stimulus is severe, compensatory mechanisms can be overwhelmed.
![]() While the exact cellular mechanisms are unknown, it appears that seizure initiation is caused by an imbalance between excitatory (e.g., glutamate, calcium, sodium, substance P, and neurokinin B) and inhibitory neurotransmission (e.g., γ-aminobutyric acid [GABA], adenosine, potassium, neuropeptide Y, opioid peptides, and galanin).13
While the exact cellular mechanisms are unknown, it appears that seizure initiation is caused by an imbalance between excitatory (e.g., glutamate, calcium, sodium, substance P, and neurokinin B) and inhibitory neurotransmission (e.g., γ-aminobutyric acid [GABA], adenosine, potassium, neuropeptide Y, opioid peptides, and galanin).13
Most of what is known has focused on gated ion channels.13 GCSE is largely caused by glutamate acting on postsynaptic N-methyl-D-aspartate (NMDA) and ε-amino-3-hydroxy-5-methyl-isoxazole-4-propionate (AMPA)/kainate receptors. During GCSE, glutamate activation of the NMDA and AMPA receptors causes opening of the gated calcium and sodium channels, which lead to neuronal depolarization. Sustained depolarization may maintain GCSE and eventually cause neuronal death through calcium-, free radical-, and kinase-mediated events.9 Although drugs acting as NMDA and AMPA receptor antagonists seem attractive, it is likely that glutamate is not the sole mechanism for GCSE and that other mechanisms become increasingly important as the duration of seizures increases.
Little is known about second messenger systems (e.g., metabotropic glutamate receptors) and the development of GCSE. GABAA postsynaptic receptors control chloride channels to produce hyperpolarization (inhibition) of the postsynaptic cell membrane. These receptors have binding sites for GABA and select anticonvulsants (e.g., phenobarbital and benzodiazepines) and enhance GABAA-mediated chloride inhibitory currents. It was previously thought that a decrease in presynaptic GABA led to prolonged seizures; however, it is currently held that GABA concentrations increase during the early phases of GCSE and continue to be elevated during late GCSE. Prolonged seizures lead to decreased inhibitory GABAA-receptor density because of postsynaptic receptor endocytosis. Additionally, modification of GABAA receptors during SE may decrease response to both endogenous GABA and GABA agonists.10 Clinically, the relative potencies of benzodiazepines and phenobarbital can be reduced up to 10-fold if seizures persist for more than 30 minutes.10 A similar phenomenon occurs with sodium channel antagonists (phenytoin); however, the magnitude of resistance is less.
PATHOPHYSIOLOGY
As GCSE persists, there are systemic alterations, progression of motor phenomena, and development of specific EEG findings.14 Two distinct and predictable phases have been identified. Phase I occurs during the first 30 minutes of seizure activity, and phase II immediately follows. Although these systemic complications affect the prognosis of GCSE, a prolonged seizure can destroy neurons independent of these events.9 In fact, the systemic effects of induced seizures in animals can be blocked, but the damage to the neocortex, cerebellum, and hippocampus persists.
During phase I, each seizure markedly increases plasma epinephrine, norepinephrine, and steroid concentrations, which can cause hypertension, tachycardia, and cardiac arrhythmias.15 Within minutes, arterial systolic pressures can rise to above 200 mm Hg, and heart rate can increase by 83 beats per minute. Mean arterial pressure does not fall below 60 mm Hg; hence, cerebral perfusion pressure is not compromised. In animals, cerebral blood flow is also increased, thereby protecting neurons from hypoxic injury.
In the presence of a hypoxic myocardium, seizure-induced increases in sympathetic and parasympathetic stimulation of the heart can result in ventricular arrhythmias. Autonomic neuron stimulation can cause a release of insulin and glucagon. Concurrently, circulating catecholamines cause an elevation of hepatic cyclic adenosine monophosphate, producing glycogenolysis. Although the patient can be hyperglycemic initially, serum glucose begins to fall.
Seizure-induced muscular contractions and hypoxia cause lactic acid release, which can produce severe acidosis that maybe accompanied by hypotension and shock. Muscle contractions can be so severe that rhabdomyolysis with secondary hyperkalemia and acute tubular necrosis can occur. The airway can be obstructed, causing the patient to become cyanotic or hypoxic. Additionally, an increase in salivation and tracheal and pulmonary secretions can cause aspiration pneumonia. Although transient pleocytosis can develop, it should not be attributed to SE until infectious causes have been eliminated. Between seizures, the EEG slows, and blood pressure normalizes. Although metabolic demands are increased, the brain is able to adequately compensate.
When seizures exceed 30 minutes (phase II), the EEG ictal discharge and clonic motor activity become continuous, and the patient begins to decompensate. Despite elevated levels of catecholamines, the patient can become hypotensive. During this time, autoregulation of cerebral blood flow becomes dependent on mean arterial pressure and begins to fail. There continues to be an excessive consumption of oxygen and glucose; however, compensatory mechanisms are no longer able to meet demands.
During Phase II, the serum glucose concentration may be normal or decreased. Profound hypoglycemia, secondary to hyperinsulinemia, can occur in those with hepatic dysfunction or reduced glycogen stores. Hyperthermia and respiratory deterioration with hypoxia and ventilatory failure can develop. Metabolic and biochemical complications, including respiratory and metabolic acidosis, hyperkalemia, hyponatremia, and azotemia, may develop. There is increased sweating and salivation.
CLINICAL PRESENTATION AND DIAGNOSIS
Accurate diagnosis requires observation, physical examination, laboratory assessment, EEG, and neurologic imaging. The nature and duration of the seizure should be obtained, but a diagnosis of GCSE should not be made until a clinician has observed a seizure. Most patients have an altered consciousness that ranges from obtunded to marked lethargy and somnolence with pronounced eyes-open unresponsiveness and waxy rigidity. Motor features can include muscle contractions, extensor or flexor posturing, and spasms. Over time, the clinical manifestations become less apparent. This has important ramifications in that seizures appear to have terminated without treatment or when an ineffective therapy is given.
In addition to an assessment of language and cognitive abilities, the physical and neurological examinations should assess motor, sensory, and reflex abnormalities, pupillary response, asymmetry, and posturing. The patient should also be examined for secondary injuries (e.g., tongue lacerations, shoulder dislocations, and head and facial trauma).
Laboratory tests are essential to the diagnosis of various etiologies. Hypoglycemia, hyponatremia, hypernatremia, hypomagnesemia, hypocalcemia, and renal failure all can cause seizures. A urine drug screen can help eliminate illicit drug use or drug overdose. Serum drug concentration(s) should be obtained in those on chronic anticonvulsants, as low concentrations can reflect partial adherence or rapid drug withdrawal. A baseline serum concentration is necessary to determine whether a loading dose of a specific anticonvulsant is required. Assessment of other laboratory parameters (e.g., hematology and chemistries to include albumin, renal function, and hepatic function) that affect anticonvulsant dosing also can be useful. An EEG is a valuable diagnostic tool, particularly in those with prolonged GCSE in whom clinically apparent seizures are not always evident, but therapy should not be delayed while awaiting testing or results.
CLINICAL PRESENTATION GCSE
< div class='tao-gold-member'>



