Squamous Carcinoma
EDI BROGI
Squamous carcinoma (SQC) of the breast is a form of metaplastic carcinoma. Because of its distinctive pathologic and clinical features, SQC is discussed here separately from all other metaplastic mammary carcinomas, including those with spindle cell and squamous differentiation, which are described in Chapter 16. The diagnosis of SQC is used for tumors in which 90% or more of the lesion consists of keratinizing SQC. By definition, a primary SQC of the breast has to be centered in the breast parenchyma. It may involve the skin only peripherally, thus excluding any primary cutaneous neoplasm that extends into the breast secondarily. Metastasis of SQC from an extramammary site should be ruled out clinically when carcinoma with squamous differentiation is encountered in the breast. The earliest examples of SQC were reported nearly a century ago.13
ORIGIN OF MAMMARY SQC
The origin of SQC of the breast remains uncertain. Cases of pure squamous ductal carcinoma in situ (DCIS) have been described, including one associated with an invasive carcinoma showing focal squamous differentiation,4 but squamous DCIS is rarely found in association with invasive SQC. It is commonly believed that the majority of pure squamous mammary carcinomas probably originate from benign squamous metaplasia5 that can occur in the epithelium of cysts,6 in hyperplastic ducts and lobules (Figs. 17.1 and 17.2), and in papillomas.7 Rarely the epithelium of multiple ducts in one portion of the breast can be extensively altered by squamous metaplasia.7,8 Squamous metaplasia is also sometimes present in fibroepithelial lesions9,10 and in gynecomastia where it is typically found as isolated foci involving part of the epithelium of ducts that also exhibit epithelial hyperplasia.11 Reddick et al.8 studied squamous metaplasia in a papilloma by using immunohistochemistry and electron microscopy and concluded that the metaplastic change originated in myoepithelial cells. Diffuse squamous metaplasia of duct and lobular epithelium in an area of fat necrosis has been described12; the patient remained well at follow-up 3 years after the biopsy. Squamous metaplasia can also be found in other inflammatory or necrotizing lesions such as infarcted adenomas,13 in inflamed cysts or other forms of mastitis, in infarcted papillomas14 and adenomyoepitheliomas (Fig. 17.3), and in healing biopsy sites (Fig. 17.4). Atypical cells obtained by fine-needle aspiration (FNA) or core biopsy from an irradiated biopsy site where there is squamous metaplasia may suggest carcinoma.15
Shousha16 described a 2 × 2 × 1 cm3 multiloculated cyst lined largely by keratinizing stratified squamous epithelium in the breast of a 70-year-old woman. Mucin-containing glandular cells were also present in the squamous epithelium, individually and in small groups (Fig. 17.5). Metaplastic squamous epithelium may become embedded in the wall of an inflamed cyst, resulting in a pattern that is difficult to distinguish from invasive carcinoma. The diagnosis usually hinges on a careful evaluation of the cytologic appearance of the squamous epithelium.
Cutaneous squamous epithelium displaced into the breast by a needle core biopsy procedure may persist in the healed tissues, resulting in the formation of an epidermal inclusion cyst.17 Squamous metaplasia of lactiferous ducts is important in the pathogenesis of subareolar abscesses.18 Chronic inflammation resulting in benign squamous metaplasia likely represents a predisposing factor to SQC.
Insulin enhances the development of squamous metaplasia in organ cultures of human mammary tissue.19,20 Chemical carcinogens cause keratinizing metaplasia in vitro in murine mammary21 and prostate glands.22 In one study, tissues obtained from patients in the early part of the menstrual cycle were less susceptible to the induction of squamous metaplasia than specimens taken later in the cycle, an observation suggesting that progesterone or estrogen, or both, may influence the process.23
CLINICAL PRESENTATION
SQC typically presents as a palpable mass. No clinical features are specific for SQC of the breast, but a substantial number of the reported cases have presented with clinical features of an abscess.24,25,26,27,28,29,30,31,32,33 Behranwala et al.34 described SQC that arose within a recurrent or long-standing breast cyst, in a chronic sinus, and in the capsule of a breast implant. Rare cases were associated with nipple discharge34,35 or Paget disease.32
All reported examples of primary mammary SQC have been in female patients. There are no documented reports in men. The mean age at diagnosis of SQC was 64 years according to California Cancer Registry data pertaining to breast carcinomas diagnosed between 1988 and 2005.36 Other
studies have reported a median age at diagnosis of 52,37 54,38 and 55,39,40 years with a range of 24 to 91 years.34,37,40,41,42 Sixty-four percent of patients diagnosed with SQC in a study from the M.D. Anderson Cancer Center were White/Caucasian, 21% African American, and 15% were of Hispanic ethnicity.37 No association of primary SQC of the breast with familial cancer syndromes has been reported. Immunosuppressive treatment with azathioprine has been associated with increased incidence of squamous cell carcinoma (SQC) of the skin and oral mucosa. One case of SQC of the breast has been reported in a 35-year-old woman treated with azathioprine for Crohn disease.43 A study found that primary mammary SQC is more common in the left breast,41 but predominant breast laterality is not mentioned in other studies.
studies have reported a median age at diagnosis of 52,37 54,38 and 55,39,40 years with a range of 24 to 91 years.34,37,40,41,42 Sixty-four percent of patients diagnosed with SQC in a study from the M.D. Anderson Cancer Center were White/Caucasian, 21% African American, and 15% were of Hispanic ethnicity.37 No association of primary SQC of the breast with familial cancer syndromes has been reported. Immunosuppressive treatment with azathioprine has been associated with increased incidence of squamous cell carcinoma (SQC) of the skin and oral mucosa. One case of SQC of the breast has been reported in a 35-year-old woman treated with azathioprine for Crohn disease.43 A study found that primary mammary SQC is more common in the left breast,41 but predominant breast laterality is not mentioned in other studies.
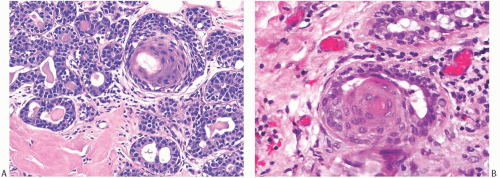
 FIG. 17.1. Squamous metaplasia in normal breast. A: A small focus of squamous differentiation is shown in one of several ducts (arrow). B: Two ducts are fully involved by benign squamous epithelium. |
Fixation to the chest wall and invasion of the skin may complicate large tumors. Extension to and ulceration of the skin can make it difficult to distinguish between cutaneous origin and secondary skin involvement by a primary mammary lesion.44 When the bulk of the tumor is in the breast and the clinical history indicates that a breast mass preceded a skin lesion, the lesion may be considered a mammary carcinoma. SQC of the breast typically presents as a mass lesion. It has indistinct or partially distinct margins on mammography, but no specific mammographic finding has been described.45,46 Calcifications in necrotic squamous tissue may be detected radiographically. The cystic nature of the tumor is usually apparent on ultrasonography.5 SQC that is cystic due to central necrosis has low T1 and high T2 signal intensity on magnetic resonance imaging (MRI).47
GROSS PATHOLOGY
SQC tend to be somewhat larger than other types of breast carcinoma. Reported sizes vary from 1 to 12.5 cm, with about 20%39 to 40%48 of the tumors measuring 5 cm or greater in
diameter. In one series, 61% of 31 pure SQC had T2 size at presentation, and 12% had T3 size.37
diameter. In one series, 61% of 31 pure SQC had T2 size at presentation, and 12% had T3 size.37
It is not unusual for the lesions to undergo cystic degeneration centrally (Fig. 17.6). This alteration is especially common in tumors larger than 2 cm, the cavity being filled with necrotic squamous and inflammatory debris.49,50 The tumor tends to be softer and more granular when the lesion is composed largely of keratinizing epithelium, whereas spindle cell metaplasia produces a firmer lesion.
MICROSCOPIC PATHOLOGY
Before establishing a diagnosis of primary SQC of the breast, it is necessary to exclude a metastasis from an extramammary primary carcinoma.40,50,51 The most common sources of metastatic SQC in the breast are the lung, uterine cervix, urinary bladder, and carcinomas of the head and neck regions.52 Although clinically the patient may be known to have an extramammary primary malignancy, this information is sometimes not communicated to the pathologist, especially if the other lesion was not treated recently and no active tumor is apparent at the primary site. Cystic degeneration is not unusual in foci of SQC metastatic from extramammary sites.
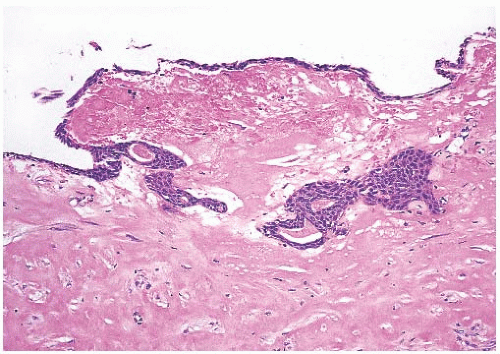 FIG. 17.4. Squamous metaplasia in biopsy cavity. A thin layer of squamous epithelium lines the surface of this partially healed biopsy cavity and a duct in the underlying tissue. |
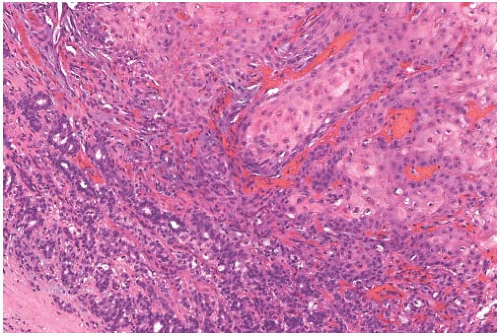
Mammary SQC are distinguished from the diverse group of metaplastic carcinomas by origin from in situ SQC in a cyst, ducts, or both, and by predominant squamous differentiation. Microscopically, they resemble SQC that arise in other sites (Fig. 17.7). Cytoplasmic clearing is present in some tumors (Fig. 17.8). Focal conversion of the squamous epithelial pattern to spindle cell pseudosarcomatous and acantholytic growth may occur53,54




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree