Splenic Preservation
Charles E. Lucas
Anna M. Ledgerwood
Dangers of the Asplenic State
Throughout the past millennia, knowledge of splenic function has been elusive. The ancients considered the spleen to be essential for such diverse functions as laughter, humor, purification of the blood, and the maintenance of life. In contrast, many senior surgeons were taught that the spleen is a vestigial organ without function. Scientific reconciliation of these conflicting opinions awaited twentieth-century investigations.
These philosophical controversies had no clinical relevance until Riegner, in 1892, introduced the modern era of splenic surgery when he performed a successful splenectomy following a blunt injury. This 14-year-old construction worker fell from a scaffold and sustained a grade V splenic injury (Table 1), with portions of the spleen lying free in the peritoneal cavity. This operation helped to establish two principles. First, laparotomy with definitive control of bleeding, rather than extraabdominal compression for refractory intraabdominal bleeding, leads to a better outcome. Second, splenectomy is safe and well tolerated, thus laying to rest the ancient concept that the spleen was necessary for life.
During most of the nineteenth century, the spleen was considered an unimportant organ. Early twentieth-century authors supported that view despite data suggesting the contrary. For example, Pearce, in 1918, noted that 25% of animals undergoing splenectomy died from either peritonitis or pneumonitis; unfortunately, these deaths were thought to be unrelated to the asplenic state as reflected in the conclusion that animals survived splenectomy with “relative impunity.” Pearce also noted that splenectomy leads to eosinophilia and leukocytosis, which were of unknown significance. Shortly thereafter, Morris and Bullock documented the importance of the spleen as an immune organ. Asplenic rats, subjected to a Pasteurella bacterial challenge, did poorly when compared to their normal controls; they suggested that the spleen was important for immunity. The surgical leadership in the Western world, however, rejected these findings by concluding that fears of infection after splenectomy were poppycock; thus, routine splenectomy for even the smallest of injuries became the norm for the ensuing generation.
Table 1 Severity of Splenic Injury | ||
|---|---|---|
|
The seed that gave rise to our current appreciation of splenic function was planted when King and Shumacker reported two deaths in five children, who died of an overwhelming postsplenectomy infection (OPSI) after splenectomy for spherocytosis. Smith, in 1957, reported the first death from OPSI following splenectomy in an injured patient without preexisting comorbidities. Subsequent studies demonstrated that OPSI occurs in about 2% of children and 1% of adults; the mortality in the non-vaccinated patient was <50%. These findings led to a reassessment of splenic function.
Splenic Function
The spleen at birth is small and remains immature throughout the neonatal period. During this phase, there are few lymphoid follicles and a relative absence of germ centers. Throughout childhood, the spleen slowly increases in size and reaches its maximal weight during puberty when it represents about 25% of the total body lymphoid mass. Perfusion through the mature spleen facilitates leukocyte sequestration and the production of both immunoglobulins
and antibodies to circulating antigens. This immunologic protection is thought to be mediated through the perifollicular lymphoid cells. The result is enhanced opsonization and phagocytosis of bacteria. The resultant enhancement of bacterial clearance is directed primarily toward the encapsulated Gram-positive bacteria, but also affects Gram-negative bacteria. These protective responses may also affect bacteria accessing the body by way of the respiratory route.
and antibodies to circulating antigens. This immunologic protection is thought to be mediated through the perifollicular lymphoid cells. The result is enhanced opsonization and phagocytosis of bacteria. The resultant enhancement of bacterial clearance is directed primarily toward the encapsulated Gram-positive bacteria, but also affects Gram-negative bacteria. These protective responses may also affect bacteria accessing the body by way of the respiratory route.
Immunological Efficiency
This immune protection appears to correlate with the amount of spleen that is present; animals with hemisplenectomy do far better than their asplenic controls, but not as well as in splenic animals when subjected to a bacterial challenge. Furthermore, the volume of the remaining spleen, following partial splenectomy, correlates directly with the antigenetic response. Thus, preservation of the maximal amount of splenic tissue affords the best protection against OPSI. The common organisms responsible for OPSI are the Gram-positive encapsulated bacteria, namely, Pneumococcus, Meningococcus, Staphylococcus, and Streptococcus; however, Escherichia coli, Haemophilus influenza, and other coliform organisms may lead to this syndrome. The intact spleen participates in the normal response to the polyvalent pneumococcal vaccine. Thus, vaccination is best administered before splenectomy.
Operative Splenic Salvage After Injury
Once the spleen became recognized as a vital link in the immune response to infection, operative attempts at splenic salvage became fashionable. A few far-sighted surgeons, however, practiced splenic salvage before their less prescient colleagues. Zikoff, in 1897, may have performed the first splenorrhaphy; Dretzka, in 1930, published the first splenorrhaphy performed on a child. This early report by Dretzka and the subsequent report by Morganstern and Shapiro highlight the technical factors involved with achieving successful splenorrhaphy. These authors emphasized the importance of a generous incision, proper exposure, and gentle manipulation when using the open approach to splenic salvage.
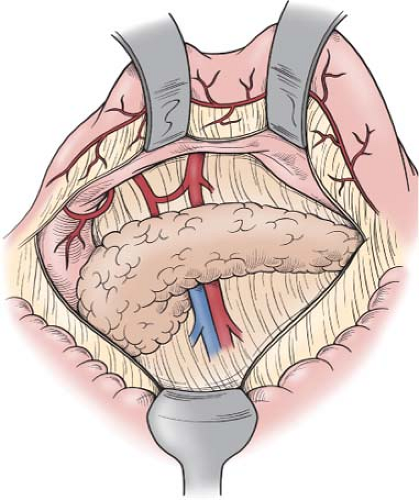
Typically, in the injured patient, this involves a midline incision, although a left subcostal incision provides excellent exposure in patients who are known to have injury limited to the left upper quadrant. The actively bleeding spleen should be packed in order to obtain temporary hemostasis while full splenic exposure is accomplished. The lesser sac is entered through the greater omentum outside the arcades of the gastroepiploic vessels (Fig. 1). As this dissection is carried superiorly along the greater curvature of the stomach, the proximal branches of the left gastroepiploic vessels may need to be divided; this facilitates the dissection near the apical fat pad and the cardiophrenic ligament. Dissection of the apical fat pad helps expose and control the highest short gastric branch. Mobilization of the proximal stomach anteriorly and medially allows one to visualize the short gastric vessels coming from the superior branches of the splenic artery as these branches enter into the superior segment of the spleen. Careful dissection of these vessels permits safe division. The splenic flexure of the colon is then mobilized. Typically, there will be two (sometimes one) short vessels extending from the colonic mesentery to the inferior pole of the spleen. These are the same “aberrant” vessels that are notoriously avulsed from the splenic capsule when too much anterior and medial tension is placed on the omentum during gastric mobilization for truncal vagotomy. These vessels are always present; they should be identified and made hemostatic by coagulation or ligation prior to division.
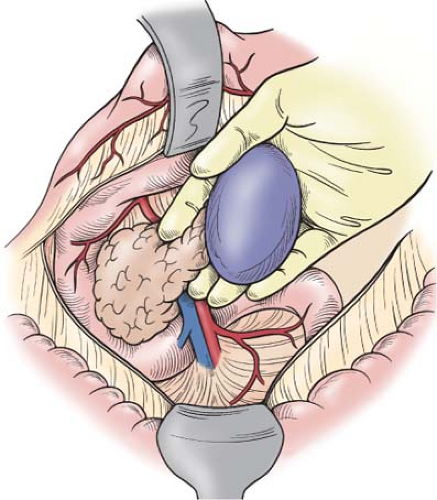
The spleen can then be detached from its “avascular” diaphragmatic and retroperitoneal attachments, remembering that small vessels are often present in these attachments; they can be controlled by electrocoagulation. Patients with portal hypertension may have extensive collaterals in this “avascular” plane, necessitating suture ligation of the peritoneal edges. This can be achieved by dividing the retroperitoneal <2 cm from the spleen between clamps and suture ligating each side. The spleen shares in this hyperdynamic, hypertensive state, thereby, making successful splenorrhaphy unlikely in cirrhotic patients. Such patients are served best by splenectomy. When bleeding occurs, placement of packs posterior to the area of digital dissection controls bleeding and allows the operation to proceed as the spleen is mobilized. The plane posterior to both the spleen and the body and tail of the pancreas is then bluntly dissected, taking care to remain within this plane and not extend posteriorly, where one might injure the adrenal vessels or renal vessels. The pancreas with
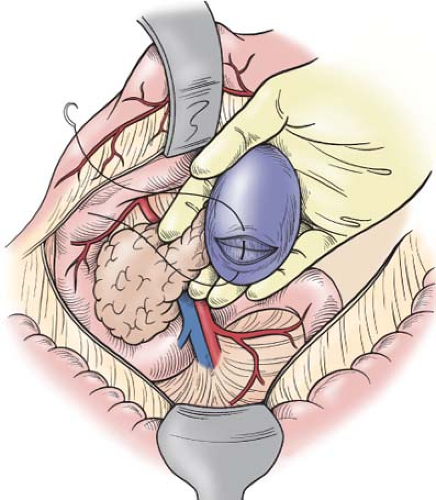
spleen is then gently pushed anteriorly and medially; one must never pull on the spleen in an effort to lift the pancreas (Fig. 2). When the injured spleen is actively bleeding, control of hemorrhage can be achieved by compressing the splenic hilum with the fingers and thumb of the left hand while the right hand is used to continue the anterior and medial mobilization of both the pancreatic body with tail and spleen. Again, packs can be placed posteriorly in the plane that was previously occupied by these organs. When digital pressure does not control splenic bleeding, the operation will likely be hurried; this almost certainly will result in the patient requiring a total splenectomy. Therefore, this tedious and compulsive mobilization with digital hemostasis should be continued until the spleen has been fully delivered to the wound (Fig. 2).
spleen is then gently pushed anteriorly and medially; one must never pull on the spleen in an effort to lift the pancreas (Fig. 2). When the injured spleen is actively bleeding, control of hemorrhage can be achieved by compressing the splenic hilum with the fingers and thumb of the left hand while the right hand is used to continue the anterior and medial mobilization of both the pancreatic body with tail and spleen. Again, packs can be placed posteriorly in the plane that was previously occupied by these organs. When digital pressure does not control splenic bleeding, the operation will likely be hurried; this almost certainly will result in the patient requiring a total splenectomy. Therefore, this tedious and compulsive mobilization with digital hemostasis should be continued until the spleen has been fully delivered to the wound (Fig. 2).
Splenorrhaphy
Once fully mobilized, there are a variety of techniques for obtaining splenic hemostasis. For large deep injuries, the authors prefer the placement of 2-0 chronic sutures swedged on a 2-in., blunt tip needle; these are placed about 1 to 2 cm away from the torn margin and passed deep into the wound crevice; the suture exit tract should be a mirror image of the entrance placement (Fig. 3). Often, two of these sutures are placed sequentially so that the surgeon can tie one while the other is held by the assistant, with the exact tension to achieve gentle compression of the spleen without tearing the parenchyma or causing tissue necrosis. Equal tension on both sides of the crevice is best obtained with two-handed ties, with each hand being close to the splenic surface. Displays of virtuosity by immature residents using fancy one-handed ties should be discouraged; these cause uneven tension, which promotes parenchymal injury with additional bleeding. Once apposed, most bleeding will rapidly subside; minor oozing through the needle holes is best treated by placing a dry sponge over the spleen and returning the spleen to the left upper quadrant for about 10 minutes while other organs are assessed. If hemostasis appears good when the pack or sponge is removed, the “no touch” technique applies; the surgeon should leave it alone.
Partial Splenectomy
When performing a partial splenectomy for extensive injury to a splenic segment, or for segmental splenic ischemia distal to transected hilar segmental vessels, transverse intraparenchymal arteries may continue to bleed. Sometimes, these transverse spurting vessels can be made hemostatic by electrocoagulation applied to an occlusive clamp, whereas other bleeding vessels require suture ligatures (Fig. 4). The authors prefer to use a 4-0 silk suture placed in a figure-eight manner to best avoid tearing of the adjacent splenic tissue while getting hemostatic occlusion of an arterial pumper (Fig. 4). The same principles of gentleness apply when suturing the splenic parenchyma surrounding a spurting vessel; a two-handed tie with equal tension reduces the chance for added parenchymal injury.
Following partial splenectomy, the splenic capsule may be approximated, especially if the splenic crevice angles toward a central valley. This approximation should be done with fine parenchymal sutures, such as 4-0 silk, and they should be tied quite gently. Bleeding through the needle holes is best contained by placing a pack over the repair and returning the spleen to the left upper quadrant, as previously indicated.
When the injury causes disruption of the hilar vessels, identification and ligation provide good hemostasis, whereas an obviously ischemic splenic segment, distal to the ligation, is best resected using the techniques described previously. There are many adjuncts to these techniques. These include the use of a variety of hemostatic agents to augment the hemostatic effect afforded by temporary packs; the argon laser may be more effective than simple electrocoagulation for larger splenic tears; the use of stapling devices gives quicker control, but may cause ischemia or tearing; and the placement of absorbable meshes around the badly injured spleen, which is then restored to the left upper quadrant and covered, may provide salvage of grade V injuries. In general, the authors advocate simplicity in providing splenic preservation; when complicated techniques must be used, the surgeon must ask the question whether the added efforts are conducive to an enhanced risk-to-benefit ratio in the patient with ongoing bleeding.
Using these techniques, the success of operative splenic salvage is 50%. The best results have been obtained in patients with blunt injury, although these techniques can be used for penetrating wounds. Attempts at operative splenic salvage are discouraged, however, in the unstable patient with active bleeding from the spleen and/or from other organs; the extra time required for splenic preservation will increase the need for blood transfusion and compromise the patient’s overall well-being.
Splenectomy with Splenic Replantation
Splenosis is a condition whereby patients with shattered spleens will sometimes develop islets of splenic growth following splenectomy when not all of the splenic fragments are identified and removed. Stimulated by this unusual event, the use of splenic preservation, by placing small fragments of splenic tissue within the folded omentum, has been promulgated. This “omental omelet” of multiple splenic fragments, measuring no more than 1 cm in size, may contain 50% of splenic parenchyma. This technique has often been used in patients requiring rapid splenectomy for bleeding after penetrating wounds. Large clinical series have demonstrated that these splenic segments remain viable and will concentrate isotope. The clinical protection against OPSI afforded by this splenic omelet, however, has not lived up to expectations. Likewise, these patients with splenic replantation do not respond in the same way as do patients with splenic salvage in the normal position with maintained splenic blood flow or in patients without splenic injury. Maintenance of normal splenic perfusion is critical to successful clearance of circulating bacteria. The paradox of having a live splenic replant without the expected immunology projected was studied in a canine of splenic replantation over 18 months; the results of this study explain why the omental omelet does not provide full immunologic protection. The splenic omelets in this canine model uniformly survive and take up isotope, but the histology of these surviving splenic fragments shows that the perifollicular lymphoid tissue is absent. This lymphoid tissue is thought, by most researchers, to be the source of the protective immune response. Based on these animal studies and bad clinical experiences, the authors no longer recommend this technique.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree