20 SPERMATOGENESIS
The male reproductive system is responsible for (1) the continuous production, nourishment, and temporary storage of the haploid male gamete (spermatozoa [sing. spermatozoon], or sperm); and (2) the synthesis and secretion of male sex hormones (androgens).
THE TESTES
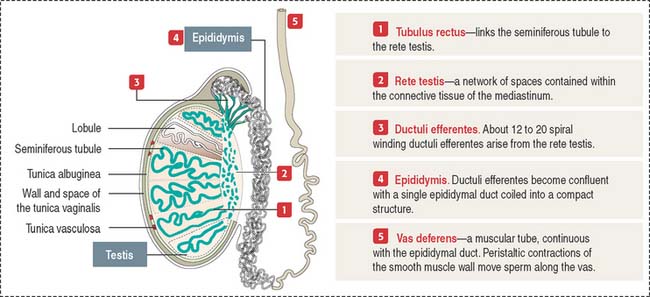
The testes is enclosed by the tunica albuginea, which is thickened to form the mediastinum where the rete testis is located (Figure 20-1). Fibrous septa from the mediastinum project into the testicular mass, dividing the tissue into 250 to 300 lobules. Each lobule contains one to four seminiferous tubules.
Each seminiferous tubule is about 150 μm in diameter and 80 cm long; it is U-shaped with the two ends opening in the rete testis. The rete testis is a network of channels that collects the products of the seminiferous epithelium (testicular sperm, secretory proteins, and ions).
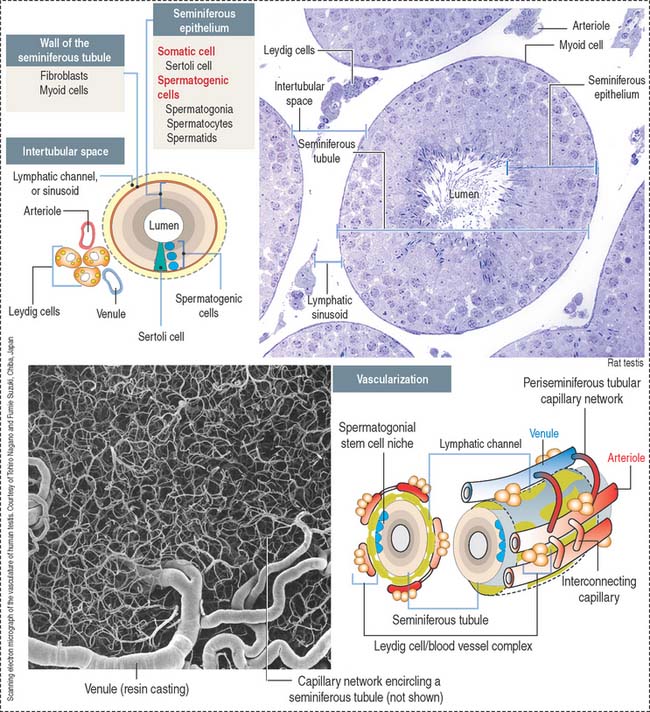
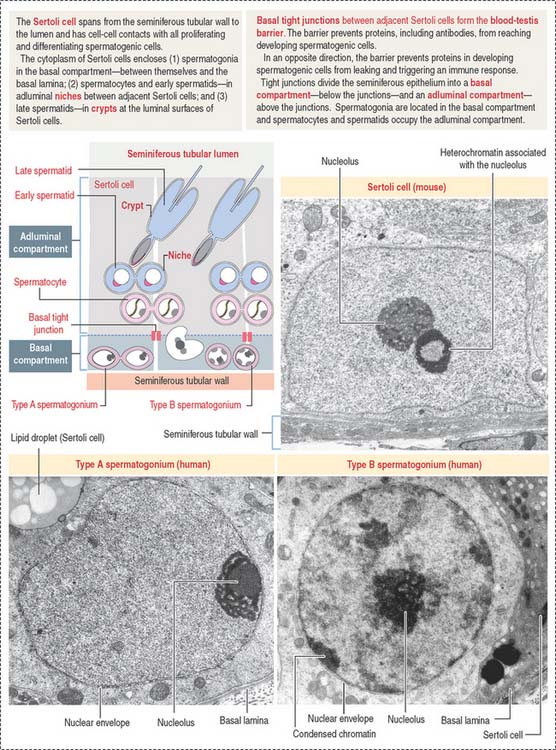
The seminiferous tubule (Figure 20-2) consists of a central lumen lined by a specialized seminiferous epithelium containing two distinct cell populations: (1) the somatic Sertoli cells and (2) the spermatogenic cells (spermatogonia, spermatocytes, and spermatids).
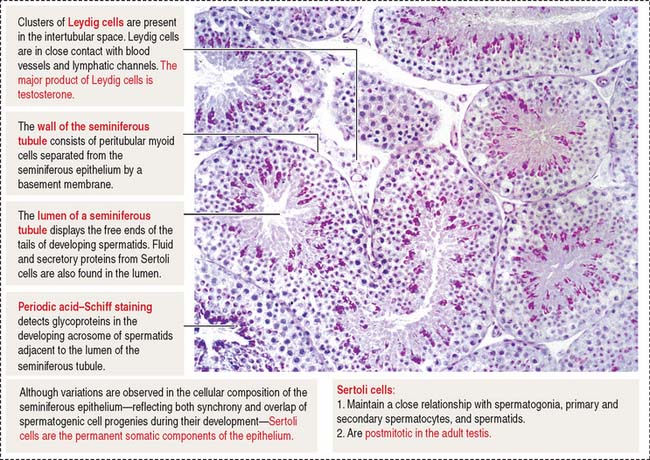
The seminiferous epithelium is encircled by a basement membrane and a wall formed by collagenous fibers, fibroblasts, and contractile myoid cells. Myoid cells are responsible for the rhythmic contractile activity that propels the nonmotile sperm to the rete testis. Sperm acquire forward motility after they have passed through the epididymal duct.
The space in between the seminiferous tubules is occupied by abundant blood vessels (arterioles, capillaries and venules) and lymphatic channels encircling each seminiferous tubule, and aggregates of the androgen-producing Leydig cells in close proximity to the lymphatic and blood circulation (see Figure 20-2). The general histologic structure of the testes is shown in Figure 20-3.
Seminiferous epithelium
Figure 20-4 illustrates relevant aspects of a mammalian spermatogenic cycle.
Sertoli cells
Sertoli cells are columnar cells extending from the basal lamina to the lumen of the seminiferous tubule (Figure 20-5). They act as bridge cells between the intertubular space and the lumen of the seminiferous tubule.
At their basolateral domain, Sertoli cells form tight junctions with adjoining Sertoli cells.
Basolateral tight junctions (1) subdivide the seminiferous epithelium into a basal compartment and an adluminal compartment (see Figure 20-5) and (2) are the determining components of the so-called blood-testes barrier, which protects developing spermatocytes and spermatids from autoimmune reactions.
ABP is a secretory protein with high binding affinity for the androgens testosterone and dihydrotestosterone. The androgen-ABP complex, whose function is unknown at present, is transported to the proximal segments of the epididymis (see Figure 20-16).
Sertoli cells secrete inhibin and activin subunits (α and β subunits). Inhibin (an αβ heterodimer) exerts a negative feedback on gonadotropin-releasing factor and FSH release by the hypothalamus and anterior hypophysis. Activin (an αα or ββ homodimer) exerts a positive feedback on the release of FSH (see Chapter 18, Neuroendocrine System).
Spermatogonia
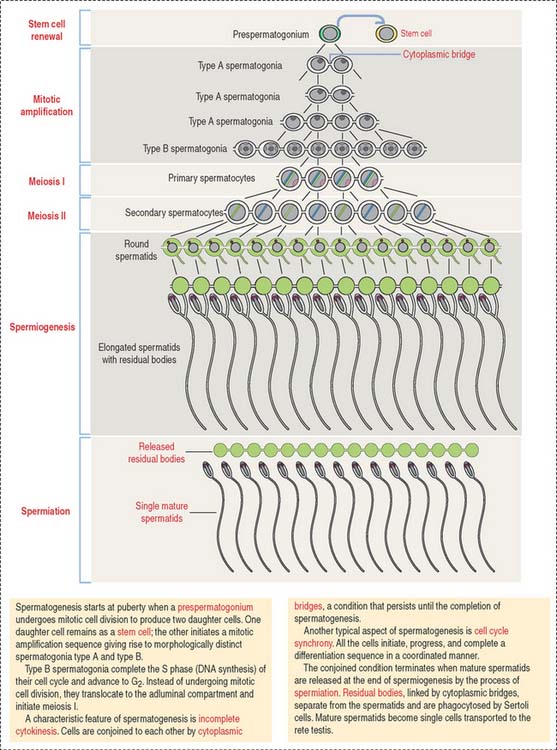
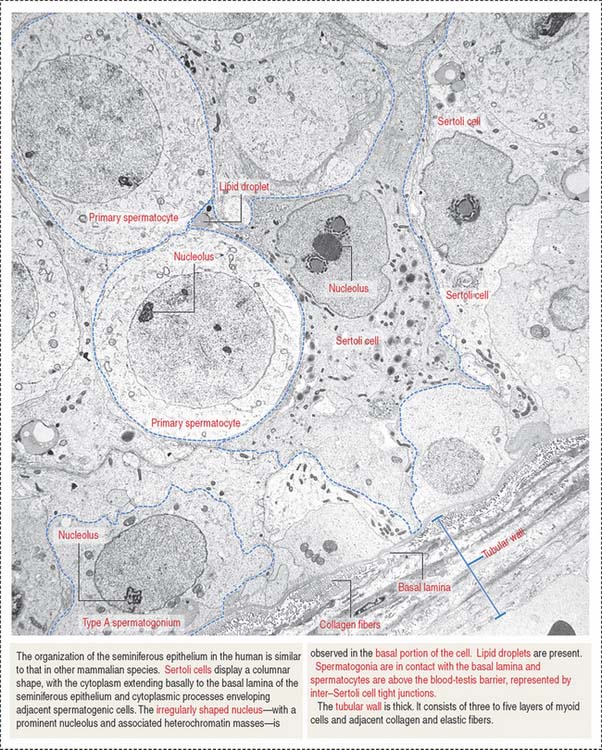
Spermatogonia are diploid spermatogenic cells directly in contact with the basal lamina in the basal compartment (Figures 20-5 to 20-7). They are located below the inter–Sertoli cell occluding junctions and therefore outside the blood-testes barrier.
Two major morphologic spermatogonial cell types can be observed:
Spermatogonial stem cells have important implications for male fertility. They are relatively quiescent and therefore resistant to radiation and cancer chemotherapy. Mitotically dividing spermatogonia, meiotically dividing spermatocytes, and differentiating spermatids are sensitive to radiation and cancer chemotherapy. After cessation of radiotherapy or anticancer chemotherapy, spermatogonial stem cells can reestablish the spermatogenic process. Postmitotic Sertoli cells are highly resistant to these therapies.
Spermatocytes
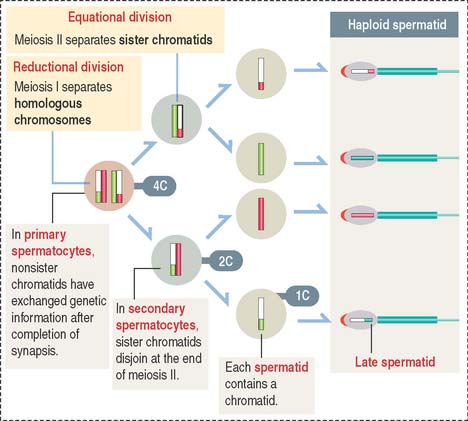
Type B spermatogonia enter meiotic prophase immediately after completing the last S phase (DNA synthesis). This last round of major DNA synthetic activity in the lifetime of spermatogenic cells determines that a primary spermatocyte starting meiotic prophase I will have two times the amount of DNA of a spermatogonium. The primary spermatocyte has a 4C DNA value, where 1C equals about 1.5 pg of DNA per cell.
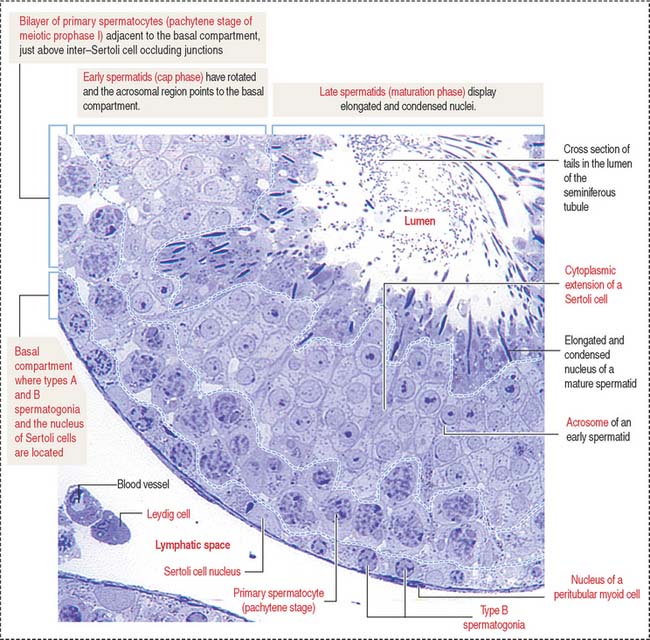
Spermatocytes divide by two successive meiotic cell divisions (Figure 20-8) and are located in the adluminal compartment of the seminiferous epithelium, just above the inter—Sertoli cell occluding junctions. Therefore, meiosis occurs inside the blood-testes barrier.
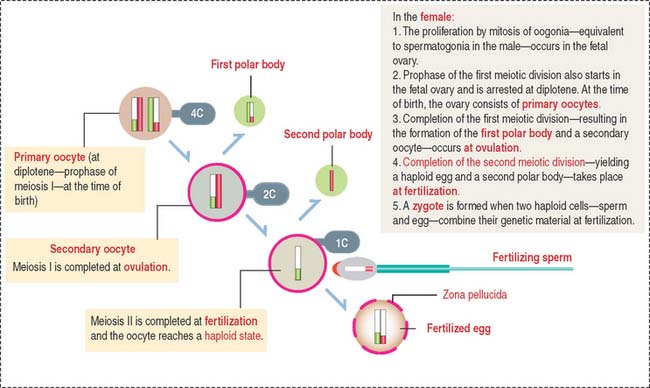
Because the first meiotic division is a long process (days) and the second meiotic division is very short (minutes), primary spermatocytes are the most abundant cells observed in the seminiferous epithelium. For comparison, Figure 20-9 illustrates the meiotic process of the female gamete that is initiated in the ovary during fetal development (see Chapter 23, Fertilization, Placentation, and Lactation).
Meiosis
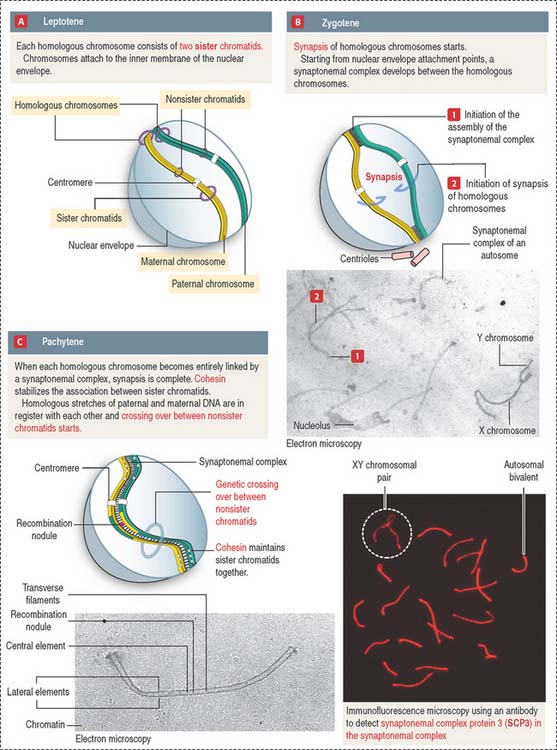
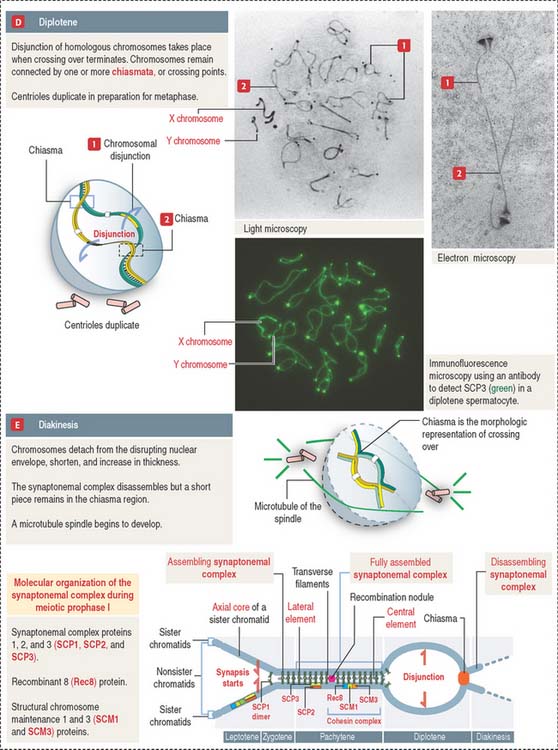
The prophase substages of the first meiotic division are the leptotene (threadlike), zygotene (pairing), pachytene (thickening), diplotene (appearing double), and diakinesis (moving apart) stages (Figures 20-10 and 20-11).
These substages are characterized by four major events: (1) the formation of a synaptonemal complex (see Box 20-A) during zygotene-pachytene to facilitate the pairing or synapsis of homologous chromosomes (autosomes and sex chromosomes X and Y); (2) the pairing of homologous chromosomes (synapsis); (3) crossing over (the exchange of genetic information between non-sister chromatids of homologous chromosomes); and (4) disjunction (the separation of paired homologous chromosomes).
Box 20-A Synaptonemal complex
In the female (see Figure 20-9), a primary oocyte (with a 4C DNA content) completes the first meiotic division at ovulation to produce a secondary oocyte (2C DNA content) and the first polar body. When fertilization occurs, the secondary oocyte completes the second meiotic division to reach the haploid state (1C DNA content), and a second polar body is generated.
Spermatids
Haploid spermatids are located in the adluminal compartment, in proximity to the seminiferous tubular lumen. There are two major types of spermatids: (1) round or early spermatids, housed in niches in the cytoplasm of Sertoli cells, and (2) elongated or late spermatids, housed in crypts, deep invaginations in Sertoli cell apical cytoplasm.
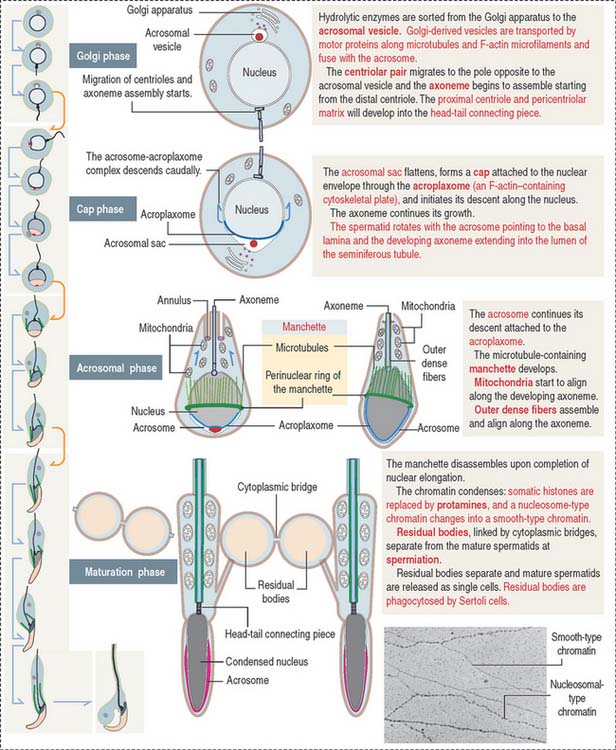
Spermatids are engaged in a highly differentiated cell process designated spermiogenesis. Spermiogenesis is the last phase of spermatogenesis. Mature spermatids are released into the seminiferous tubular lumen by a process called spermiation. Spermiation involves cytoskeletal contractile forces generated at the apical ectoplasmic region of Sertoli cells.
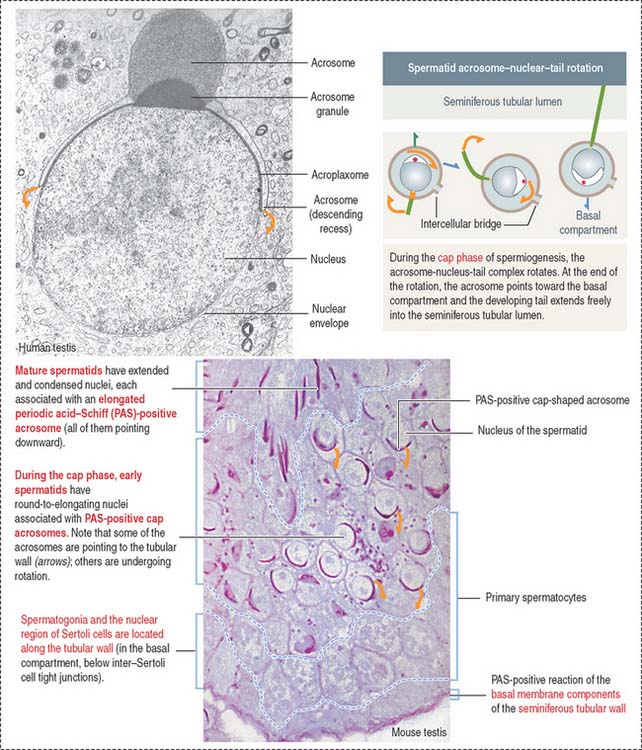
Four major events characterize spermiogenesis (Figures 20-12 and 20-13):
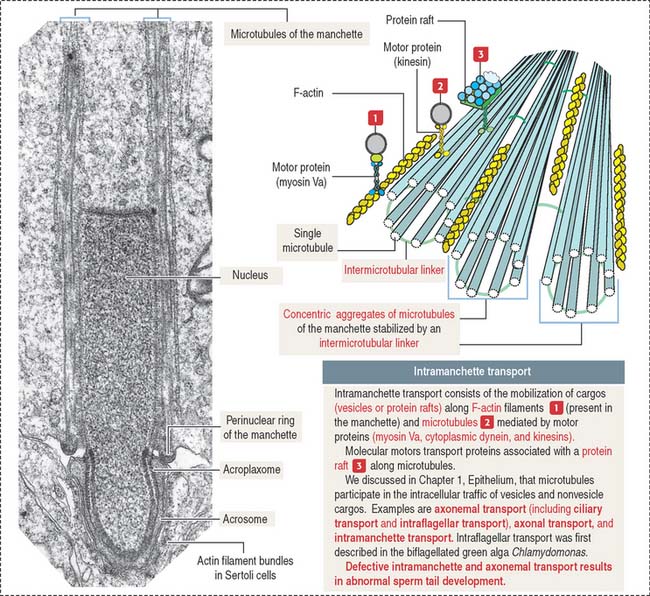
The sperm flagellum is a complex structure. It is formed by the axoneme (9 + 2 micro tubule doublets in a concentric arrangement) surrounded by mitochondria forming a helicoidal sheath around the proximal segment of the tail (called the middle piece), and outer dense fibers. The distal segment of the tail, called the principal piece, consists of the axoneme surrounded by outer dense fibers, a pair of ribs, and a fibrous sheath. An annulus, which contains the protein septin 4, demarcates the middle-to-principal segment transition of the sperm tail (Figure 20-14). A lack of septin 4 causes male sterility.
The development of the acrosome consists of four sequential phases: the Golgi phase, cap phase, acrosomal phase, and maturation phase (see Figures 20-12 and 20-13). The acrosome is required for fertilization.