21 SPERM TRANSPORT AND MATURATION
DEVELOPMENT OF THE GONADS
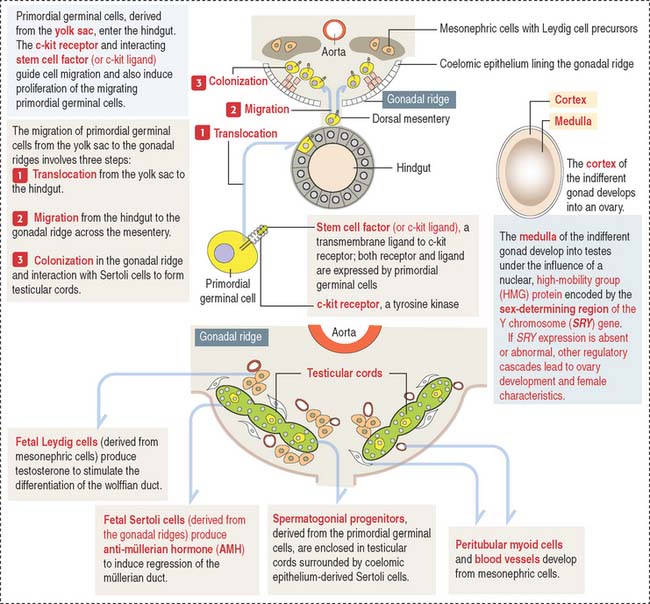
An important aspect to keep in mind is that the cell precursors of both gametes have an extra-embryonic origin. Primordial germinal cells (PGCs) appear first in the endoderm of the yolk sac wall in the 4-week fetus (Figure 21-1).
The migration and proliferation of primordial germinal cells are dependent on the interaction of the c-kit receptor, a tyrosine kinase, with its corresponding cell membrane ligand, stem cell factor (or c-kit ligand). Both the c-kit receptor and stem cell factor are produced by primordial germinal cells along their migration route.
Role of the anti-müllerian hormone and testosterone in the development of male and female internal genitalia
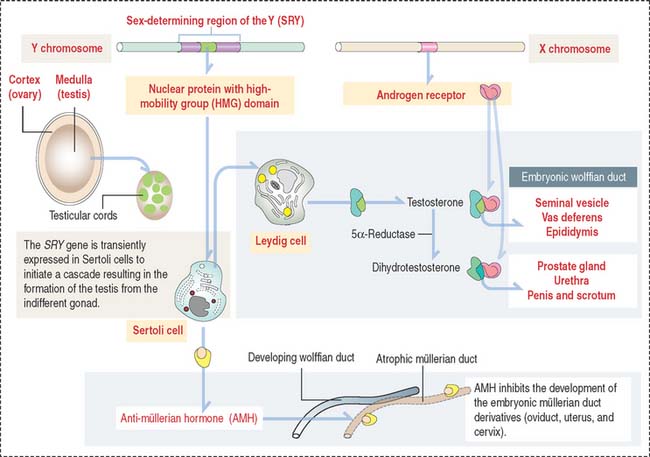
Fetal Sertoli cells secrete anti-müllerian hormone (AMH), which prevents müllerian ducts (also called paramesonephric ducts) from developing into the uterovaginal primordium (Figure 21-2). In the absence of AMH, the müllerian ducts persist and become the female internal genitalia.
In the absence of androgen, the wolffian duct regresses and the prostate fails to develop. If high levels of androgen are present in the female fetus, both müllerian and wolffian ducts can persist (see Box 21-A).
Box 21-A Development of internal genitalia
Testicular descent
The gubernaculum forms on the lower pole of the testis, crosses obliquely through the abdominal wall, and attaches the testis to the scrotal swelling. By week 28, the testis moves deep into the inguinal ring. The gubernaculum grows and the testis descends into the scrotum. For additional details, see Cryptorchidism (or undescended testis) in Chapter 20, Spermatogenesis.
Clinical significance: Klinefelter’s syndrome
The excess of estradiol can lead to phenotypic feminization, including gynecomastia.
Clinical significance: Androgen insensitivity syndrome (testicular feminization)
At puberty, the production of both androgen and estradiol increases (the latter from peripheral aromatization of androgens). Androgens cannot inhibit LH secretion (because a defective androgen receptor prevents LH feedback inhibition), and plasma levels of androgens remain high.
SPERM MATURATION PATHWAY
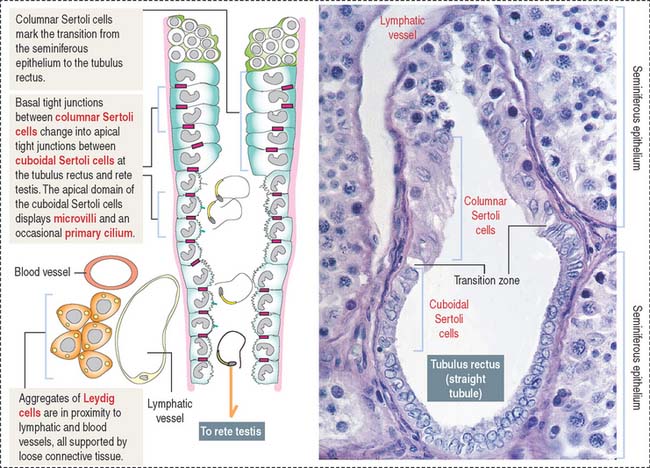
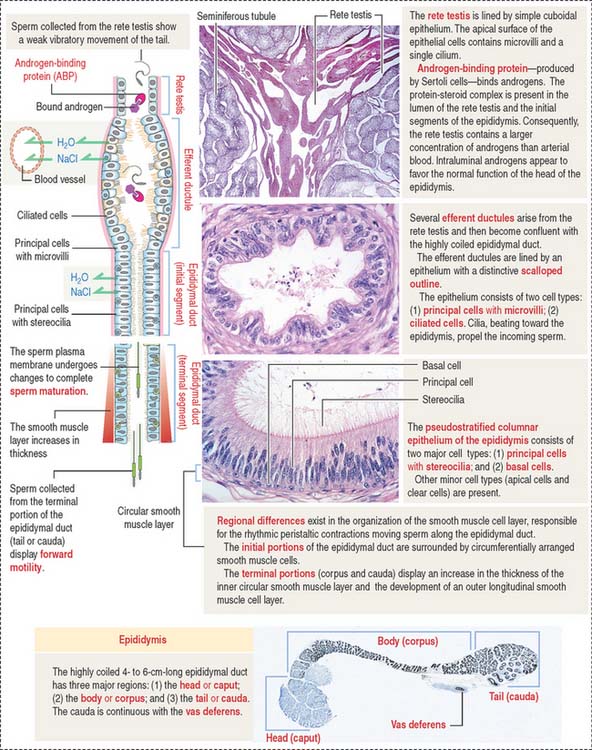
After transport to the rete testis through a connecting tubulus rectus (Figure 21-3), sperm enter the ductuli efferentes. Ductuli efferentes link the rete testis to the initial segment of the epididymal duct, an irregularly coiled duct extending to the ductus, or vas deferens.
The rete testis consists of irregularly anastomosing channels within the mediastinum of the testis (Figure 21-4). These channels are lined by a simple cuboidal epithelium. The wall, formed by fibroblasts and myoid cells, is surrounded by large lymphatic channels and blood vessels associated with large clusters of Leydig cells.
About 12 to 20 ductuli efferentes (efferent ductules) link the rete testis to the epididymis after piercing the testicular tunica albuginea. Each ductule is lined by a columnar epithelium with principal cells with microvilli—with a role in the reabsorption of fluid from the lumen—and ciliated cells, which contribute to the transport of nonmotile sperm toward the epididymis. The epithelium has a characteristic scalloped outline that enables identification of the ductuli efferentes (see Figure 21-4). A thin inner circular layer of smooth muscle cells underlies the epithelium and its basal lamina.
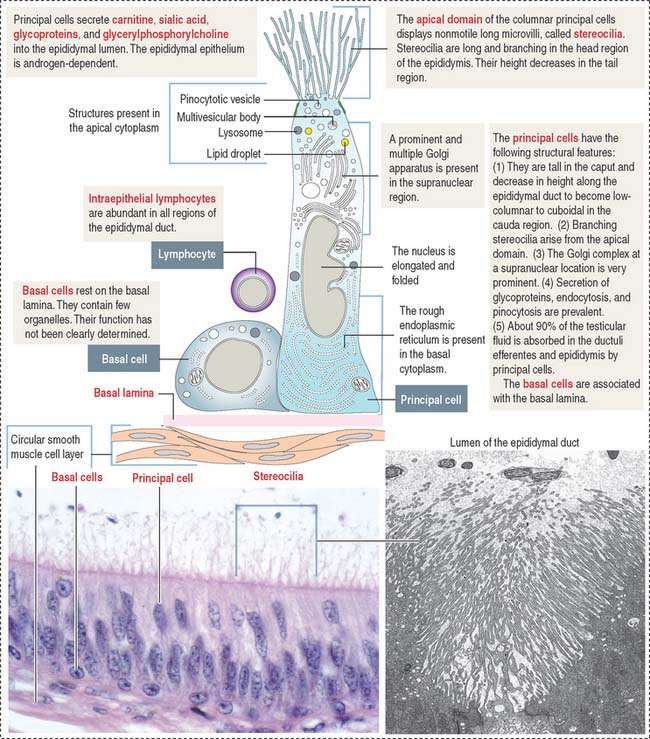
The epididymal duct is subdivided into three major segments: (1) the head or caput; (2) the body or corpus; and (3) the tail or cauda (see Figure 21-4).
The epithelium is pseudostratified columnar with long and branched stereocilia. The epithelium consists of two major cell types (Figure 21-5):
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree