Chapter 2 Specialized techniques in dermatopathology
Specimen fixation, grossing/ put-through, processing, embedding and sectioning
The aim of fixation in dermatopathology is to maintain clear and consistent morphological features and to preserve tissue in an optimal state suitable for a range of staining and ancillary histopathological techniques.1,2 Most fixation methods employed during tissue processing depend on chemical fixation of tissue in liquid fixatives.3 Tissue fixation may also be accomplished by physical (heat, microwave, freeze-drying and freeze substitution) and/or chemical (coagulant and cross-linking) methods.4 The most commonly used fixative is 10% neutral-buffered formalin solution. The quality of fixation is affected by:
Formalin fixation occurs at an approximate rate of 1 mm per hour.4,6,7 The volume of the fixative should be at least 10 times the volume of the specimen.7 Large specimens, such as tumors, may require sectioning into 5-mm thick slices, covering with fixative soaked gauze or cloth and fixation overnight.5,7
Diagnostic dermatological biopsies may be:
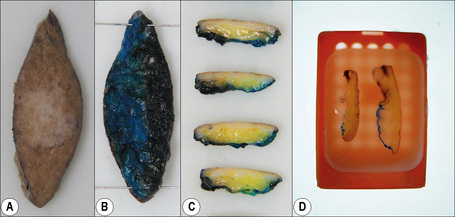
Prior to put-through, excisional specimens that require an appraisal of margins should be inked. If localization sutures have been inserted by surgeons then four-quadrant, four-color painting or two-color painted halves (Fig. 2.1) may be appropriate. Shave biopsies are used to sample or remove lesions, and if of appropriate size, may be divided into sections, bisected or trisected and embedded on edge. Edge embedding is critical in a shave excision of a lesion such as a small melanoma so that the width and depth of invasion can then be quantified.8,9 The main purpose of core or punch biopsies, which generally measure 2–8 mm in diameter, is to sample large lesions. Biopsies larger than 4 mm in size should be bisected eccentrically and the specimens embedded with the cut surfaces down. The eccentric sectioning ensures that the lesion is not missed. Biopsies less than 4 mm are put through in toto.9,10
Tissue processing refers to a series of steps that effect the removal of extractable water from biopsies to ensure sections of optimal diagnostic quality.9 These include fixation, dehydration, clearing, infiltration and embedding in a support matrix. Use of manual and automated tissue processing achieves this goal, including:
In most laboratories, overnight processing runs are the norm.9 However, microwave-assisted tissue processing facilitates shorter processing times of one to two hours. Dehydrating reagents promote the removal of unbound water and aqueous fixatives from the tissue. Clearing reagents serve as an intermediary between the dehydrating and infiltrating solutions, being miscible with both. Paraffin is the most popular infiltration and embedding medium, being suitable for the majority of routine and special stains. The important principle to be adhered to during embedding of skin biopsies is that the orientation of the skin sample should offer the least resistance to the blade during microtomy. Skin biopsies are usually cut in a plane at right angles to the epidermis so that the epidermal surface is sectioned last, minimizing its compression and distortion.
Fig. 2.2 Technical artifact: folds in tissue sections because of poor water bath floating technique.
Routine and ‘special’ stains
Diagnostic sections are usually stained with hematoxylin and eosin (H&E), the most widely used routine stain.1 The hematoxylin component stains the nuclei blue-black and the eosin stains the cytoplasmic compartment and connective tissue in variable shades and intensity of pink, orange and red. The periodic acid-Schiff (PAS) technique is used widely to demonstrate:
The PAS technique is therefore employed to demonstrate basement membrane thickening in lupus erythematosus, porphyria cutanea tarda and in some tumors. Glycogen is digested by diastase, while neutral mucopolysaccharides are not. Mucicarmine demonstrates acidic epithelial mucins.2 It is useful for the diagnosis of adenocarcinomas and the mucoid C. neoformans capsule. Alcian blue highlights acidic mucopolysaccharides, staining the mucinous components of dermal mucinoses, granuloma annulare, scleredema of Bushke, lupus erythematosus and metastatic adenocarcinomas. Alcian blue demonstrates heterogeneity of staining that is pH based: sialomucins are demonstrated at pH 2.5 and sulfamucins at pH 1.0.3
While colloidal iron, initially described by Hale for the identification of acid mucopolysaccharides, is as sensitive as Alcian blue for this purpose, its specificity and selectivity are debatable and background staining may be problematic.4 However, reduction of pH of the colloidal iron solution and inclusion of acetic acid washes may reduce this artifact.3–5 The high iron diamine stain, in contrast to colloidal iron, stains highly acidic sulfamucins but does not stain sialomucins or hyaluronic acid.5–7 Connective tissue stains highlight collagen, elastic and reticulin fibers. The trichrome stain, a combination of three dyes, is employed for the differential demonstration of muscle, collagen fibers, fibrin and erythrocytes.8 Elastic fibers may stain with eosin, phloxine, Congo red and PAS stains but are demonstrated well with the Verhöeff method in the diagnosis of scleroderma, anetoderma and pseudoxanthoma elasticum. Silver stains are useful to demonstrate reticulin fibers, melanin and the identification of infective agents. While methenamine silver and Gomori Grocott methenamine silver stains highlight fungi and bacteria, Warthin-Starry, Dieterle and Steiner silver stains are particularly useful in the demonstration of spirochetes, B. henselae and Donovan bodies (Fig. 2.5). Masson-Fontana silver staining is pivotal to the staining of the cell wall of C. neoformans, especially in the identification of capsule-deficient C. neoformans. The role of the more commonly used special stains is summarized in Table 2.1.
Immunohistochemical techniques
Since the first practical application of antibodies using the peroxidase labeled antibody method on paraffin-embedded tissues in 1968, immunohistochemistry (IHC) has emerged as a powerful supplementary investigation to histomorphologic assessment.1–3 IHC has widespread dermatopathologic diagnostic, prognostic, therapeutic and pathogenetic applications, not only in a range of neoplastic (Table 2.2), immunobullous and infective disease, but also in the distinction between reactive and neoplastic disorders.4–14 Immunohistologic techniques can be performed manually or in automated platforms. While automation allows enhanced quality and reproducibility of staining, detailed, exact IHC protocols are critical in the many laboratories that still perform manual IHC, to achieve optimal, reproducible results.
Table 2.2 Some diagnostic immunohistochemical applications for cutaneous tumors4–13
| Stain | Application |
|---|---|
| Epidermal and appendageal neoplasms | |
| AE1/AE3 | Pan-keratin. Confirms epithelial lineage |
| CAM 5.2 | CKs 8,18. Confirm epithelial lineage. Useful to confirm glandular neoplasms |
| MNF 116 | CKs 5, 6, 8, 17, 19. Useful in diagnosis of SCC with single cell infiltration |
| BerEP4 | Positive in BCC. Negative in SCC. |
| CK 7 | Confirmation of mammary and extra-mammary Paget’s disease |
| p63 | Distinguish primary cutaneous spindle SCC from mesenchymal spindle cell tumors & primary cutaneous adnexal from metastatic adenocarcinomas |
| CD10 | Trichoepithelioma: positive in stroma and papillae, negative in epithelium. BCC: positive in epithelium, negative in stroma. |
| bcl2 | Positive in BCC, negative in SCC. |
| Vascular proliferations | |
| CD31 | High specificity and good sensitivity for endothelial tumors |
| CD34 | High sensitivity but low specificity for endothelial tumors |
| Fli-1 | Nuclear staining of endothelial tumors |
| GLUT 1 | Positive in endothelial cells of all juvenile hemangiomas. Usually negative in congenital hemangiomas (rapidly involuting congenital hemangioma and non-involuting congenital hemangioma) |
| Melanocytic tumors | |
| S-100 protein | Most widely used melanocytic marker. It is highly sensitive but not as specific as other melanocytic markers |
| HMB 45 | Good specificity but relatively low sensitivity. Tends to be negative in spindle cell melanoma. Also positive in PEComa. |
| Melan A/Mart 1 | Similar specificity to HMB45. Tends to be negative in spindle cell melanomas. |
| Ki-67 | Higher proliferation index in melanoma (13–35%) than in nevi (<5%). Useful in the evaluation of some melanocytic tumors, mainly nevoid melanoma |
| Neuroectodermal and neural tumors | |
| S-100 protein | Positive in neuroectodermal, neuronal, nerve sheath, chondroid tumors, some sweat gland tumors and myoepithelioma |
| NSE | Merkel cell carcinoma |
| CK 20 | Merkel cell carcinoma |
| Neurofilament | Merkel cell carcinoma |
| Chromogranin | Merkel cell carcinoma |
| Synaptophysin | Merkel cell carcinoma |
| TTF1 | Negative in most Merkel cell carcinoma |
| Myogenic/myofibroblastic differentiation | |
| MSA | Tumors of muscle origin |
| Desmin | Tumors of muscle origin (smooth muscle and skeletal muscle, rarely and focally in myofibroblastic tumors) |
| Myogenin | Positive in rhabdomyosarcoma |
| SMA | Positive in smooth muscle tumors, glomus tumor, myopericytoma, dermatomyofibroma |
BCC, basal cell carcinoma; SCC, squamous cell carcinoma; SMA, anti-smooth muscle actin; MSA, muscle specific actin; CK, cytokeratin.
Immunohistochemical techniques and trouble shooting
In many centers, IHC is now the most commonly utilized ancillary test for clinical tissue samples.
Historically, the introduction of enzymes as labels in IHC overcame difficulties associated with immunofluorescence, including the inability to assess histomorphology with the latter.1 The peroxidase-antiperoxidase (PAP) technique, was replaced by alkaline phosphatase-antialkaline phosphatase (APAAP) techniques and avidin-biotin labeling.1,2 Although the streptavidin-biotin labeling system gained popularity, the endogenous biotin-associated background staining under certain circumstances has resulted in increasing use of labeled polymer-based detection systems, suitable for manual and automated IHC platforms (Fig. 2.6).3
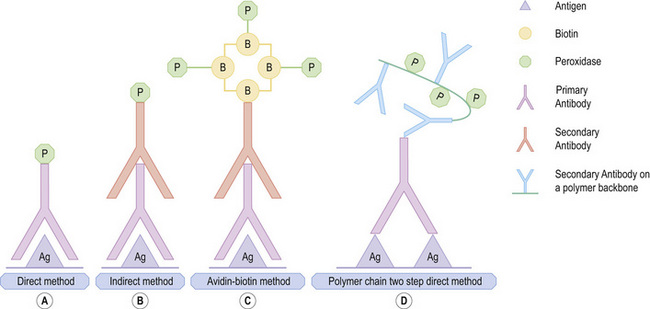
Fig. 2.6 Immunohistochemical techniques: (A) direct, (B) indirect (C) streptavidin biotin (D) polymer chain.
By courtesy of Dr. J. Deonarain, Department of Anatomical Pathology, National Health Laboratory Service, Durban, South Africa.
The direct conjugation of the primary antibody to the label formed the principle of the initial, traditional direct technique, in which the labeled antibody reacted directly with the tissue antigen.1 In the two-step indirect technique, labeled secondary antibody directed against the immunoglobulin of the animal in which the primary antibody was raised was used to visualize an unlabeled primary antibody.4 The labeled streptavidin-biotin (LSAB) method is a three-step technique. An unconjugated primary monoclonal or polyclonal antibody, attached to the tissue antigen forms the first layer, creating an antigen-antibody complex. The second layer is formed by a biotinylated secondary antibody raised against the same species of the primary animal.1 The secondary antibody binds to the primary antibody with the biotinylated end being available for binding to a third layer. This layer may bind either to enzyme-labeled streptavidin or to a complex of enzyme-labeled biotin and streptavidin. The enzyme may be horseradish peroxidase or alkaline phosphatase. An appropriate chromogen is used for detection. In the peroxidase method, peroxidase-oriented chromogens such as diaminobenzidine or 3-amino-9 ethylcarbazole are appropriate. Indole reagents (red), naphthol fast red (red) or NBT / BCIP (blue) are the chromogens used in the alkaline phosphatase-streptavidin method.1,4
The presence of endogenous biotin and resultant background staining led to the introduction of the increasingly popular polymer-based immunohistochemical methods. In the new direct Enhanced Polymer One Step (EPOS) technique, approximately 70 enzyme molecules and 10 primary antibodies are conjugated to a dextran ‘backbone’. While the entire IHC procedure is completed in one step, the method is limited to highly select manufacturer-specific primary antibodies. Other newer polymer detection systems with a dextran backbone to which multiple enzyme molecules may attach are available for manual and automated IHC. These quick, reliable and reproducible techniques are also characterized by greater sensitivity. Single-, dual-, and triple-color staining with different chromogens is possible.1,2,4,5
Background staining is a common difficulty that has multiple predisposing causes.6 While monoclonal antibodies reduce non-specific background staining, not only must antibody concentrates and prediluted preparations be optimized for usage at the correct dilution in different laboratories (Figs 2.7 and 2.8), diluent pH is also critical in ensuring the absence of antibody degeneration and resultant background staining. Avidin-biotin detection systems and horseradish peroxidase systems may require biotin blocking and endogenous peroxidase quenching steps to decrease unnecessary background staining. Polymer-based detection systems can effectively eliminate biotin-induced false-positive staining. While antigen retrieval techniques are critical for antigen unmasking, optimal results require control of the pH and temperature of retrieval solutions and controlled enzymatic digestion (Fig. 2.9).7–10 The latter causes excessive background staining when sections are exposed to increased digestion time, inappropriate high temperature and inadequate rinsing, causing protein diffusion into or deposition in skin sections and background staining.
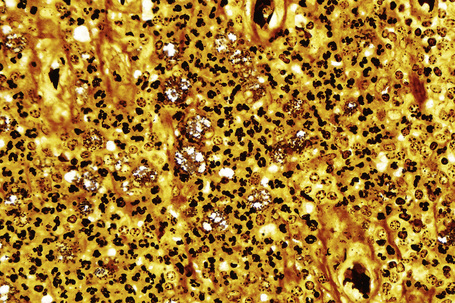
Chromogen entrapment, precipitation and contaminants may lead to false-positive interpretation of an IHC test. Depletion of peroxidase or alkaline phosphatase chromogenic activity, a consequence of the breakdown of chromogens because of the sensitivity to light and heat, results in a background blush. A similar effect is seen when there is inadequate chromogen rinsing or prolonged chromogen time. Filtering of the chromogen is effective in preventing chromogen precipitation. Chatter, tears, folds and wrinkles and poor adhesion of sections to slides causes entrapment and suboptimal rinsing of chromogen (Fig. 2.10). Skin sections with a thick stratum corneum, dermal calcification, or sclerosis may be prone to these artifacts, requiring meticulous microtomy to prevent its occurrence. The handling of water baths, tissue sections and slides with ungloved hands may cause contamination of sections with squames.1
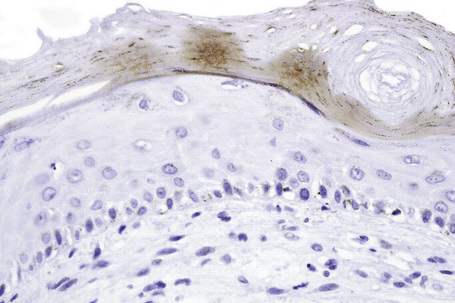
Fig. 2.10 Technical artifact: HHV8 stained sections demonstrating chromogen entrapment in stratum corneum.
Immunofluorescence
Immunofluorescent techniques have the potential to define antigen-antibody interactions at a subcellular level.1 This interaction requires the irreversible binding of a readily identifiable label for its recognition.1,2 Fluorochromes such as rhodamine or fluorescein are labels that can absorb radiation in the form of ultraviolet or visible light.1–5 Direct and indirect immunofluorescence (IMF) techniques demonstrate a range of tissue antigens of dermatopathologic importance, including the diagnosis of infectious and autoimmune blistering disorders.3 In the direct IMF technique, antibody is conjugated directly with a fluorochrome and is used to detect an antigen in a tissue section using ultraviolet light microscopy.1–3 In the indirect IMF technique, patient serum (containing the antibodies) interacts with a tissue section containing the antigen. Antibody to a human immunoglobulin, conjugated to a fluorochrome, is applied thereafter.1–7 The successful demonstration of the antigen requires the antigen to remain sufficiently insoluble in situ. Skin biopsies for direct immunofluorescence can be transported fresh on saline-soaked gauze in a container on ice, or in a transport medium such as Michel medium.8 The transport medium must be maintained at a pH of 7.0 to 7.2.1,3,5 The main uses for IMF in dermatopathology are in the interpretation of the autoimmune blistering diseases, lupus erythematosus, and vasculitis.6,7 In general, immunofluorescence has the following advantages over immunohistochemistry:
Electron microscopy
Transmission electron microscopy offers better resolution than light microscopy.1 To optimize this, tissue has to be embedded in extremely rigid material to allow sectioning at 80 nm. In most circumstances, hydrophobic epoxy resins are preferred. When a specimen is removed for ultrastructural examination, it must be fixed in a suitable fixative immediately. The volume of the fixative should be 10 times the sample size. The final specimen size is 1 mm2.1 Fixation is affected by:
Intercellular junctions, Weibel-Palade bodies, melanosomes, and premelanosomes may help in the diagnosis of carcinomas, endothelial tumors, and melanocytic tumors, respectively.3 In CADSIL, extracellular, electron-dense granular material is present in an indentation in vascular smooth muscle cells.5,6 Amyloid is identifiable as randomly arranged, extracellular, nonbranching fibrils of indeterminate length and 7–10 nm diameter.7 Transmission electron microscopy remains a valuable tool in the ongoing evaluation of the structure of normal and pathological human cell and tissue components and infective agents.8–10
Diagnosis of inherited skin diseases
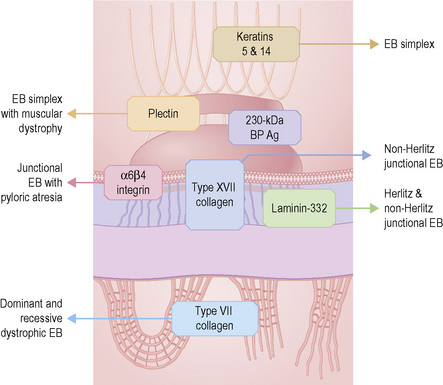
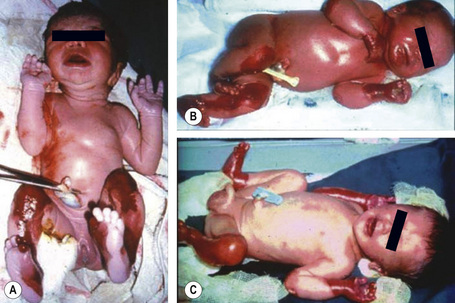
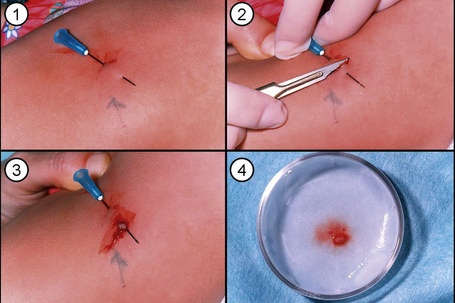
Analysis of the inherited skin blistering disorder known collectively as epidermolysis bullosa (EB) discussed in detail in Chapter 4 demonstrates the complex, multifaceted approach to diagnosis required in such cases. EB has been shown to result from mutations in genes encoding at least 11 different structural proteins at or close to the dermal–epidermal junction (Fig. 2.11).1 Clinically, the different types of EB are characterized by widely differing prognoses, from death in early infancy to blistering that may become milder in later life.2 The clinical presentation in neonates, however, can be confusing to dermatologists and pediatricians because of the overlapping features (Fig. 2.12). In these circumstances, skin biopsy, usually a superficial shave biopsy since the key region is the dermal–epidermal junction, can provide critical diagnostic and prognostic information. Typically, nonblistered skin from any body site is sampled. Just before the biopsy is taken, the skin is rubbed gently in an attempt to induce fresh microsplits at the dermal–epidermal junction, to facilitate the microscopic subtyping of EB (Fig. 2.13).
The most informative investigation is immunolabeling of the dermal–epidermal junction using a panel of basement membrane antibodies. Skin biopsies can be transported in Michel’s medium to a diagnostic laboratory at ambient temperature: this fixative is extremely useful since basement membrane zone immunoreactivity is maintained for at least 6 months.3 For the immunolabeling, frozen skin sections are used rather than formalin-fixed paraffin-embedded material because the antigenic epitopes of several transmembranous proteins may be lost in routine skin processing. The basement membrane antibodies can be used either to determine the level of cleavage in the skin (antigen mapping) or to see if there is a reduction or absence of immunostaining for a particular antigen.4Figure 2.14, for example, demonstrates labeling using an antibody against type IV collagen in skin from the neonate illustrated in Figure 2.12a. In this example, labeling maps to the roof of the split. This indicates that the lamina densa is in the blister roof and that there is a sublamina densa plane of blister formation. These findings support a diagnosis of dystrophic EB. This diagnosis can be refined by immunolabeling with an antibody to type VII collagen, as shown in (Fig. 2.15). In normal skin there is bright, linear labeling at the dermal–epidermal junction; however, in the skin from the neonate shown in Figure 2.12a, there is a complete absence of type VII collagen immunoreactivity. All other antibodies show normal reactivity at the dermal–epidermal junction. These findings therefore establish a diagnosis of severe, generalized recessive dystrophic EB. Reduced or absent immunolabeling with specific basement membrane antibodies is an extremely useful and rapid means of diagnosing recessive forms of EB. For example, skin from the neonate shown in Figure 2.12c demonstrated a lack of reactivity against laminin-332 but normal immunostaining for all other antibodies. These findings establish a diagnosis of Herlitz junctional EB.
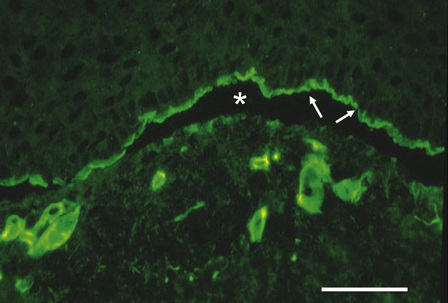
Fig. 2.14 Antigen mapping to diagnose the subtype of inherited EB: this picture shows immunolabeling of rubbed skin from an individual with EB (case illustrated in Fig. 2.12a) with an anti-type IV collagen antibody. Rubbing the skin induces microsplits at the dermal–epidermal junction (asterisk). The type IV collagen reactivity maps to the roof of the dermal–epidermal junction (arrows). This indicates a sub-lamina densa plane of cleavage and establishes a diagnosis of dystrophic EB. (Bar = 25 μm.)
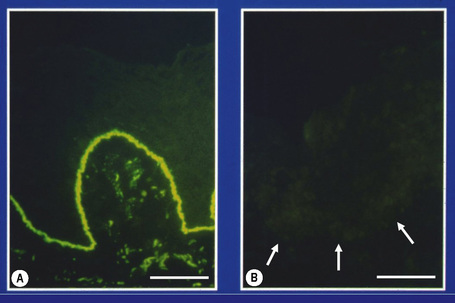
Fig. 2.15 Specific antibody probes to subtype inherited EB: (A) immunostaining of normal control skin with an antibody to type VII collagen shows bright linear labeling at the dermal–epidermal junction; (B) in contrast, the complete absence of labeling in skin from an individual with EB (case illustrated in Fig. 2.12a) indicates a diagnosis of severe, generalized recessive dystrophic EB. (Bar = 50 μm.)
The development of a panel of basement membrane antibodies, most of which are commercially available, has led to decreased emphasis on transmission electron microscopy as a diagnostic tool in EB.5 Ultrastructural analysis, however, can be useful in confirming the plane of cleavage and in establishing the diagnosis of certain dominant forms of EB. Skin from the neonate illustrated in Figure 2.12b, for example, shows normal intensity basement membrane zone reactivity for all diagnostic probes but transmission electron microscopy (Fig. 2.16) identifies discrete clumps of tonofilament and basal keratinocyte cytolysis, characteristic of the Dowling-Meara variant of EB simplex. For recessive forms of EB, however, immunolabeling of basement membrane proteins has become the most important diagnostic approach.6,7 Reduced or absent staining for a particular protein provides a rapid diagnosis as well as a means of identifying the encoding gene (or genes) in which the underlying pathogenic mutations are present. Thus the skin biopsy findings, both histologic and immunohistochemical, provide a direct guide to molecular screening tests, most of which are PCR-based, as discussed below. This molecular information can then be used for genetic counseling, carrier screening, and DNA-based prenatal testing, if indicated.
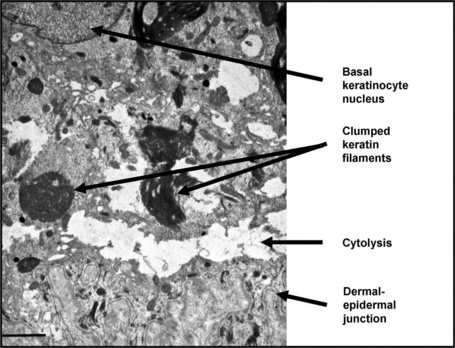
Fig. 2.16 Transmission electron microscopy of skin in Dowling-Meara EB simplex (case illustrated in Fig. 2.12b): within the basal keratinocyte cytoplasm the keratin filaments are condensed and form clumps and there is cytolysis that occurs just above the dermal–epidermal junction. (Bar = 1 μm.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree