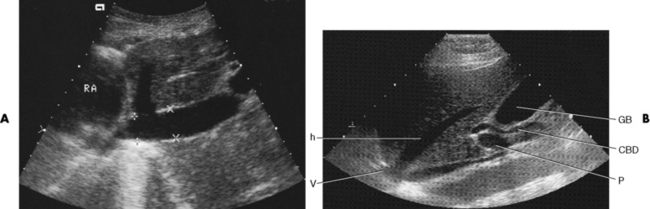
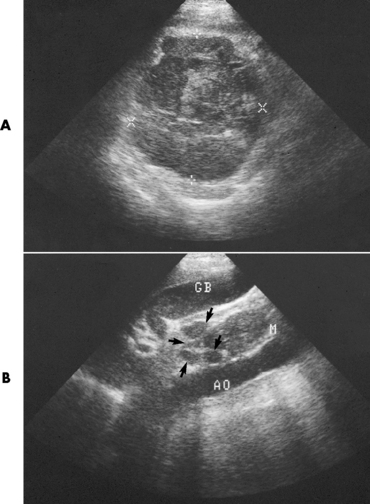
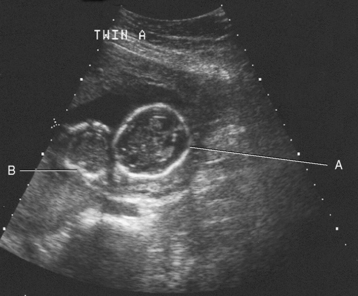
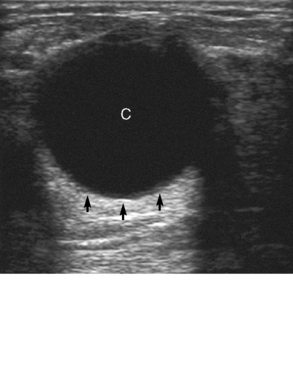
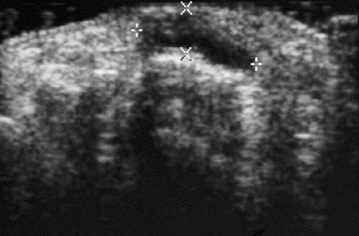
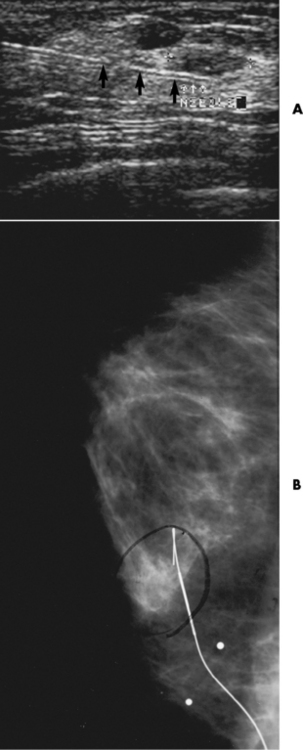
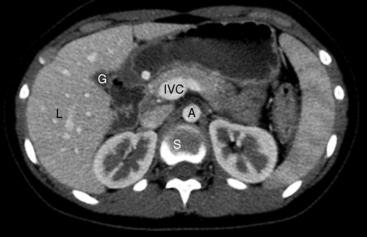
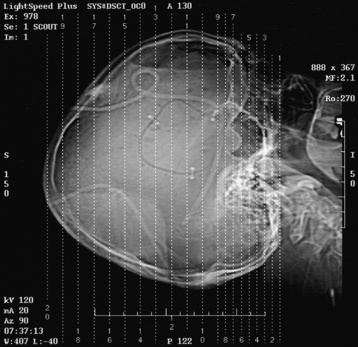
Chapter 2 After reading this chapter, the reader will be able to: 1 Differentiate screening and diagnostic mammography imaging protocols and how the protocols are used to demonstrate pathology 2 Describe the theory of image production with ultrasound and why this modality becomes the optimal choice to demonstrate pathologic conditions 3 Describe the theory of image production with computed tomography (CT) and the body structures best demonstrated 4 Briefly describe the theory of image production with magnetic resonance imaging (MRI) and the different sequences used to demonstrate specific tissue 5 Describe the theory of image production with nuclear medicine, single-photon emission computed tomography (SPECT), and positron emission tomography (PET) 6 Identify the fusion imaging techniques required to produce optimal quality images in patients with various underlying pathologic conditions. anatomic structures. With continuing research, nuclear medicine expanded its role by adding movement and a computer that allowed more than anterior and posterior projections, resulting in the development of single-photon emission computed tomography. Additional research developments in radiopharmaceuticals led to the creation of a positron-emitting radionuclide, which resulted in the newest modality—positron emission tomography. Now the concept of multiplanar imaging and gamma camera movement (tomography) has provided healthcare with two new perspectives in molecular imaging. Most modern imaging departments have a separate area where breast imaging procedures are performed. The most common imaging technique for diagnosing breast cancer is full-field digital mammography (FFDM). Some centers still use the conventional screen-film imaging, which employs a specially designed x-ray screen that permits the proper exposure of film by many fewer x-rays than would otherwise be necessary. This procedure produces a conventional black-and-white image at a very low radiation dose. Full-field digital mammography relies on radiation captured by multiple cells that convert the radiation energy to electrical energy to produce a numerical value (i.e., a digitized image). The advantages of digital mammography are faster image acquisition with lower dose (shorter exposure), increased contrast resolution with the ability to manipulate images to visualize specific areas of interest, decreased need to repeat studies, and the ease of sharing images with other professionals. Screening mammography consists of two images of each breast, the craniocaudal and mediolateral oblique projections. For a woman with a palpable nodule, the first choice may be a diagnostic mammogram, which includes an additional 90-degree mediolateral projection. When screening mammography demonstrates a suspicious area or a definite abnormality, additional images, such as coned-down or magnification projections, can be completed to compliment the study. In some cases, ultrasound supplements mammography images by demonstrating the lesion to be fluid filled (cystic) or solid. Ultrasound (also called ultrasonography) is a widely accepted cross-sectional imaging technique because of its low cost, availability, and ability to differentiate cystic (gallbladder), solid (liver), and complex (liver tumor) tissue. A noninvasive imaging modality, ultrasound uses high-frequency sound waves produced by electrical stimulation of a specialized crystal (Figure 2-1). When the high-frequency sound waves pass through the body, their intensity is reduced by different amounts depending on the acoustic properties of the tissues through which they travel. The crystal mounted in a transducer sends the signal and also acts as a receiver to record echoes reflected back from the body whenever the sound wave strikes an interface between two tissues that have different acoustic properties. The transducer records the tiny changes of the signal’s pitch and direction. A water-tissue interface can produce strong reflections (echoes), whereas a solid tissue mass that contains small differences in composition can cause weak reflections. The display of the ultrasound image on an imaging monitor shows both the intensity level of the echoes and the position in the body from which they were scanned. Ultrasound images may be displayed as static gray-scale images or as multiple (video) images that permit movement to be viewed in real time. Color display on a sonogram is used to detect motion (most specifically, blood flow). Depending on the equipment used, the interactions of the tissue with the sound wave determine how the tissue or organ is visualized and described. In general, fluid-filled structures have intense echoes at their borders, no internal echoes, and good transmission of the sound waves. Anechoic tissue or structures (which are echo free, or lacking a signal) transmit sound waves easily and appear as the dark region on the image; examples are the gallbladder and a distended urinary bladder. Solid structures (e.g., liver, spleen) produce internal echoes of variable intensity. The terms hyperechoic and hypoechoic are used to make comparisons of echo intensities between adjacent structures. For example, the normal liver can be described as being hyperechoic to the normal renal cortex because the hepatic parenchymal tissue appears as a lighter shade of gray. Conversely, because the normal renal cortex appears as a darker shade of gray than the normal liver parenchyma, it can be described as being hypoechoic to the liver. The term isoechoic is used to describe two structures that have the same echogenicity even though the tissue may not be the same; for example, liver tissue is often isoechoic to the spleen. Complex tissue types have both anechoic and echogenic areas (Figure 2-2). The major advantage of ultrasound is its safety. There has been no evidence of any adverse effect on human tissues at the intensity level currently used for diagnostic procedures. Therefore, ultrasound is the modality of choice for examinations of children and pregnant women in whom a potential danger exists from the radiation exposure involved with other imaging studies. Ultrasound is by far the best technique for evaluating fetal age and placenta placement, congenital anomalies, and complications of pregnancy (Figure 2-3). Abdominal ultrasound is used extensively to evaluate the intraperitoneal and retroperitoneal structures, to detect abdominal and pelvic abscesses, and to diagnose obstruction of the biliary and urinary tracts. Pelvic ultrasound images of the prostate gland aid in the detection and accurate staging of neoplasms. Pelvic imaging is performed via a transabdominal (through the abdominal wall), transvaginal (through the vagina), or transrectal (through the rectum) approach. Other uses of ultrasound include breast imaging (to differentiate solid from cystic masses) (Figure 2-4), musculoskeletal imaging (to detect problems with tendons, muscles, and joints, and soft tissue fluid collections or masses) (Figure 2-5), and as an imaging guide for invasive procedures (biopsies, aspirations, and drain placement) (Figure 2-6). The major limitation of ultrasound is the presence of acoustic barriers, such as air, bone, and barium. For example, air reflects essentially the entire ultrasound beam, so that structures beneath cannot be imaged well. This special problem interferes with imaging of the solid abdominal organs (e.g., the pancreas) in a patient with adynamic ileus, and it is the major factor precluding ultrasound examination of the thorax. For an ultrasound examination of the pelvis, the patient usually drinks a large amount of fluid to fill the bladder, thus displacing the air-filled bowel from the region of interest. More information on ultrasound imaging can be found on the following websites: www.aium.org, www.sdms.org and www.ardms.org. Computed tomography (CT) produces cross-sectional tomographic images by first scanning a slice of tissue from multiple angles with a narrow x-ray beam, then calculating a relative linear attenuation coefficient (representing the amount of radiation absorbed in tissue for the various tissue elements in the section), and finally displaying the computed reconstruction as a gray-scale image on a imaging monitor. Unlike other imaging modalities (except for the more recent MRI), CT permits the radiographic differentiation of a variety of soft tissues from each other (Figure 2-7). CT is extremely sensitive to slight (1%) differences in tissue densities; for comparison, detection by conventional screen-film radiography requires differences in tissue density of at least 5%. Thus, in the head, CT can differentiate between blood clots, white matter and gray matter, cerebrospinal fluid, cerebral edema, and neoplastic processes. The CT number (Hounsfield number) reflects the attenuation of a specific tissue relative to that of water, which is arbitrarily assigned a CT number of 0 and appears gray on the image. The highest CT number (1000) represents bone, which appears white, and the lowest CT number (−1000) denotes air, which appears black. Fat has a CT number less than 0, whereas soft tissues have CT numbers higher than 0. The use of the computer allows the image to be manipulated by adjustment of the window width (gray scale—contrast scale) and window level (density or brightness). From the radiographer’s perspective, the window width determines the number of densities that can be visualized on the monitor. The window level is the midpoint or center of the total number of densities being viewed in a selected window width. Predetermined window widths and window levels are used to demonstrate specific parts of the anatomy (lung, liver, bone). Technical improvements in CT instrumentation and tube heat unit capacity have greatly reduced the time required to produce multiple slices (1 to 2 seconds), permitting the CT evaluation of virtually any portion of the body. In most instances, some type of preliminary image is obtained (either a radiograph or a CT-generated image) for localization, the detection of potentially interfering high-density material (metallic clips, barium, electrodes), and correlation with the CT images. An overlying grid with numeric markers permits close correlation between the subsequent CT scans and the initial scout image (Figure 2-8).
Specialized Imaging Techniques
Diagnostic imaging modalities
Mammography
Ultrasound
Computed Tomography
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Specialized Imaging Techniques