Special Comment: the Unfinished Legacy of Liver Transplantation
Thomas E. Starzl
Noriko Murase
Legacy: Something immaterial, as a style or philosophy, that is passed from one generation to another. Anything handed down from, or as from, an ancestor.
During the last 30 years, the philosophy and practice of hepatology have been dramatically transformed by the wide acceptance of orthotopic liver transplantation. The extent that this has occurred is exemplified by the gratifying 1-, 3-, and 5-year survival after liver replacement described in the chapter by Dr. Busuttil. Nevertheless, the steady mortality rate during the first 5 postoperative years and beyond has been a persistent reminder that liver transplantation has not yet been fully optimized. The delayed losses have largely resulted from the ravages of long-term immunosuppression.
How to lighten the burden of immunosuppression requires an understanding of the full 55-year history of liver transplantation. Numerous milestones in the development of the procedure, including its first successful use in humans, were reached between 1955 and 1980 (Table 1). However, the immune response to hepatic allografts could not be controlled reliably enough to permit the wide use of liver replacement until the advent of cyclosporine in 1980. Even then, diffusion of the complex new multidisciplinary enterprise of liver transplantation into the national and international health care systems would be a prodigious task (Table 1). This was evident in the 2004 Action Plan for Liver Disease Research that was designed to “coordinate research efforts (to treat hepatic and biliary disease) across the NIH.”
Research and Development Opportunities
The National Institutes of Health (NIH) 2004 plan was divided into 16 chapters, one of which was devoted exclusively to liver
transplantation, with primary emphasis on clinical research. The chapter on “Liver Transplantation” began with the simple declarative sentence, “Liver transplantation is now the standard of care for patients with end stage liver disease or acute liver failure.” It was a proud statement from the government agency whose unfailing support had made this possible. But, had liver transplantation matured so completely that there is nothing left to do but fine-tuning? This view was negated by links to liver transplantation in almost all of the 15 other chapters of the NIH prospectus. Most of these links were to targets of research opportunity that already had been enriched by, or even owed their provenance to, liver transplantation.
transplantation, with primary emphasis on clinical research. The chapter on “Liver Transplantation” began with the simple declarative sentence, “Liver transplantation is now the standard of care for patients with end stage liver disease or acute liver failure.” It was a proud statement from the government agency whose unfailing support had made this possible. But, had liver transplantation matured so completely that there is nothing left to do but fine-tuning? This view was negated by links to liver transplantation in almost all of the 15 other chapters of the NIH prospectus. Most of these links were to targets of research opportunity that already had been enriched by, or even owed their provenance to, liver transplantation.
For example, techniques of liver procurement, preservation, and replacement have been adapted for nontransplant purposes (e.g., for subtotal hepatic resections). The discovery that portal venous blood contains substances important for maintenance of liver size, function, and the capacity for regeneration was the beginning of the still-evolving special field of hepatotrophic physiology that is concerned with the functional and hormonal interrelationships of the different splanchnic organs. The hepatotrophic studies ultimately led to the cure or palliation with liver replacement of numerous hepatic-based inborn errors of metabolism, providing the first examples of what might be accomplished in the future with gene therapy and the application of stem cell biology. Finally, religious beliefs, concerns about medical ethics, and public policy or legal issues that surfaced 5 decades ago with the first attempts of liver transplantation remained as unresolved agenda items in the NIH master plan of 2004, and today.
However, the most frequently identified potential research initiatives in the NIH strategic plan of 2004 concerned the immune response and/or the manifold consequences of modifying it, not only for transplantation, but also in the context of hepatitis, human immunodeficiency virus (HIV), and oncology (to which separate chapters of the plan were devoted). Using today’s sophisticated tools (particularly those of molecular biology), the time has come to expand the sphere of immunology in new directions, fill in knowledge gaps, explain long-standing enigmas, and contribute ultimately to better patient care. With this in mind, the following discussion will consider specific issues of immunology that are central to further development of liver transplantation and to improvement of treatment under multiple nontransplant circumstances.
Table 1 Milestones of Liver Transplantation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The Relation of Alloengraftment to Acquired Immune Tolerance
The Historical View
Bone Marrow Transplantation
Transplantation immunology was brought to its present state by a series of events that began in 1943 to 1944 with Medawar’s demonstration that rejection is an immune response. A year later, Owen discovered mixed blood cell chimerism in freemartin cattle whose fused placentas had permitted fetal cross-circulation; such animals were subsequently shown to be mutually tolerant. Then, in 1953 to 1955, the strong association of donor leukocyte chimerism and acquired donor-specific tolerance was demonstrated in experiments in which allogeneic spleen and bone marrow cells were transplanted without immunosuppression into immunologically immature mice and into irradiated adult mouse recipients. After hematolymphopoietic cell engraftment, the recipients could accept all other donor tissues and organs. The mouse tolerance models escalated during the ensuing 15 years to clinical bone marrow transplantation in immunodeficient and irradiated patients. However, success depended on the use of HLA-matched donors. Otherwise, the penalty for engraftment was lethal graft-versus-host disease (GVHD): that is, rejection of the host by the graft.
Organ Transplantation
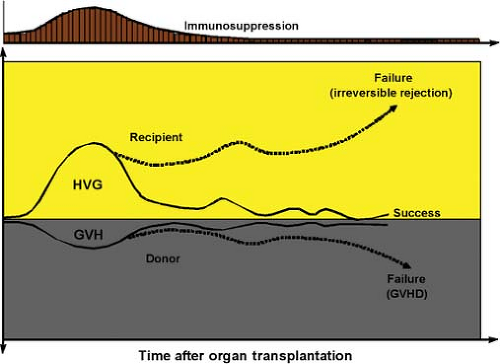
In contrast to the “bench to bedside” chronology of bone marrow transplantation, organ transplantation (initially of the kidney) was accomplished in humans before proof of feasibility was demonstrated in an animal model and in the apparent absence of leukocyte chimerism. The first six kidney recipients with prolonged graft survival (1959 to 1962) were preconditioned with sublethal total-body irradiation, but were not infused with donor bone marrow cells. In 1960 to 1961, daily posttransplant azathioprine was shown to prolong kidney survival in dogs, and taken to clinical trials. Used alone or in combination with other cytotoxic agents, azathioprine was only marginally effective. However, its combination with prednisone made renal transplantation a practical service by exposing two features of the alloimmune response that later were demonstrated with liver and all other kinds of organ transplantation and under all other regimens of immunosuppression.
The first unexplained observation was that rejections that developed under azathioprine were easily reversed with the addition of large doses of prednisone rather than being inexorable, as previously thought. Secondly, a successful rejection reversal frequently was succeeded by a greatly reduced requirement for maintenance immunosuppression (Fig. 1), suggesting that the graft was inherently tolerogenic. It also was learned that histocompatibility matching was not a prerequisite for success, that there was little threat of GVHD, and that perpetuation of organ graft survival almost always depended on lifetime drug treatment. In addition to these striking differences from bone marrow transplantation, none of the organ recipients were thought to have donor leukocyte chimerism (Table 2).
Because of these striking disparities, organ engraftment and successful bone marrow transplantation were considered for many years to involve fundamentally different mechanisms. Experimental therapeutic strategies were empirically developed with the objective of endowing organ recipients with the donor leukocyte chimerism-associated mechanisms of the bone marrow recipient while avoiding the penalty of GVHD. These strategies had in common the infusion of donor hematolymphopoietic cells into organ recipients that had been immunologically weakened by irradiation, antilymphoid antibody preparations, or other means (i.e., nonmyelotoxic cytoreduction). Although encouraging experimental results have been reported, such protocols have not found a significant niche in clinical organ transplantation practice because of their complexity, risks, and unpredictable consequences.
Nevertheless, this body of experimental work demonstrated that the establishment of a hematolymphopoietic population composed of donor and recipient cells was possible in some models with a reasonably low risk of GVHD and could result in donor-specific tolerance, providing the donor cell contribution was at least 1% to 2% (“macrochimerism”). Levels below this (“microchimerism”) were generally interpreted as either negative findings or artifacts. By so doing, the historical paradigm that attributed bone marrow and organ engraftment to different mechanisms required no substantive revision.
A Unification of Bone Marrow and Organ Transplantation
The historical paradigm was not challenged until 1992. When small numbers of multilineage donor hematolymphopoietic cells
(microchimerism) were found in animal and human recipients of long-surviving kidney and liver allografts, it was postulated that the mechanisms of organ engraftment differed only in degree from the leukocyte chimerism-dependent ones of successful bone marrow transplantation. Organ engraftment was now defined as a variable form of tolerance that resulted from “responses of coexisting donor and recipient immune cells, each to the other, causing reciprocal clonal expansion followed by peripheral clonal deletion.” The graft-versus-host arm of the double-immune reaction (the inverted curve in Fig. 1) usually was clinically inapparent.
(microchimerism) were found in animal and human recipients of long-surviving kidney and liver allografts, it was postulated that the mechanisms of organ engraftment differed only in degree from the leukocyte chimerism-dependent ones of successful bone marrow transplantation. Organ engraftment was now defined as a variable form of tolerance that resulted from “responses of coexisting donor and recipient immune cells, each to the other, causing reciprocal clonal expansion followed by peripheral clonal deletion.” The graft-versus-host arm of the double-immune reaction (the inverted curve in Fig. 1) usually was clinically inapparent.
Table 2 Differences Between Clinical Organ Transplantation and Bone Marrow Transplantation | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
Table 3 Mechanisms of Immune Nonreactivity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
The prerequisite for the donor-specific tolerance was migration of the graft’s passenger leukocytes to host lymphoid organs and induction there of the host-versus-graft response. Because the multilineage passenger leukocytes of an organ are of bone marrow origin, their hematogenous migration into the recipient was, in essence, the equivalent of a bone marrow cell infusion. Exhaustion and deletion of the antidonor response explained the characteristic rejection reversal and subsequent decline in need for immunosuppression in organ recipients (Fig. 1). Cytoablation of bone marrow, but not of organ recipients, was the apparent reason for essentially all of the differences between the two kinds of transplantation, including the high risk to the bone marrow recipient of GVHD, and the need to restrict marrow donors to those with a histocompatibility match (Table 2). Importantly, essentially all cytoablated bone marrow recipients have a small residual population of their own hematolymphopoietic cells (i.e., mirror image microchimerism) rather than complete bone marrow replacement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree