TERMS
•Biologic
•Immunity
•Multiple-dose vial
•Units
OBJECTIVES
Upon completion of this chapter, the technician student will be able to:
•Discuss drugs ordered in units and micrograms per milliliter of activity.
•Calculate insulin doses accurately.
•Calculate heparin doses accurately.
•Calculate doses for other medications based on micrograms per milliliter using a chart.
The potency of some endocrine products, vitamins, a few antibiotics, and biologics (e.g., vaccines) is based on their activity, and it is expressed in terms of units (of activity), in micrograms per milligram (μg/mg), or in other standardized terms of measurement. These measures of potency meet standards approved by the U.S. Food & Drug Administration and are set forth in the United States Pharmacopoeia (USP). In general, they also conform to international standards (e.g., international units or IU).
unit A specific quantity regarded as a whole for calculation, as a gram or a liter. Also, a term used to express activity of a drug product: USP (United States Pharmacopeia) units of activity for an antibiotic are based on a comparison of activity of a sample of that antibiotic on a milligram basis to the corresponding USP reference standard.
Measures of degrees of activity, as units of activity, are determined by comparison against a suitable working standard, generally a USP reference standard. Reference standards are authentic specimens used as comparison standards in compendial tests and assays. The number of USP units of an antibiotic, for example, is based on a comparison of activity of a sample of that antibiotic on a milligram basis to the corresponding USP reference standard. For instance, there are 1590 USP units of penicillin G sodium per milligram of the USP reference standard of the antibiotic. Pharmaceutical products and preparations are allowed specific variances in potency; for example, the USP monograph for sterile penicillin G sodium specifies a potency of 1500 to 1750 penicillin G units per milligram. The activity or potency of antibiotics is determined by their inhibitory effect on microorganisms. No relationship exists between the unit of potency of one drug and the unit of potency of another drug.
The potency of antibiotics may also be designated in terms of micrograms of activity. This concept originated when reference standards for antibiotics were thought to consist entirely of single chemical entities and were therefore assigned potencies of 1000 mcg/mg. As newer methods of antibiotic manufacture and purification were developed, it was determined that some highly purified antibiotics had greater than 1000 mcg of activity per milligram compared to the original reference standard. Differences in potency between the chemical base and the salt form were also found. For example, ampicillin sodium has a potency equivalent to 845 to 988 mcg/mg of its parent compound, ampicillin.
A comparison of units and micrograms of potency of some official drugs and their respective weight equivalents is given in Table 11.1.
 Insulin and Penicillin
Insulin and Penicillin
Just as potencies of certain drugs are designated in units, the doses of these drugs and of their preparations are also measured in units. Of the drugs for which potency is expressed in units, insulin, heparin, and the penicillin antibiotics are perhaps the most commonly used. In the case of insulin, although the commercial types vary according to time of onset of action, time of peak action, and duration of action, all products are standardized to contain either 100 or 500 insulin units per milliliter of solution or suspension. These strengths are designated as U-100 and U-500. As noted in Chapter 3, dispensing errors can occur when a medication order abbreviates the term units with a U following the number of units, since a poorly written U can be mistaken for a zero (e.g., 100U may be mistaken to be 1000 units). JCAHO regulations now prohibit the use of the abbreviation U; therefore the word units must be spelled out in JCAHO institutions (e.g., 100 units or 100 Units). Special syringes are available for measuring units of insulin, and the required dose is measured in milliliters or directly in units, depending on the calibration of the syringe. Table 11.2 shows the various insulin preparations and their onset of action (see also Figure 11.1).
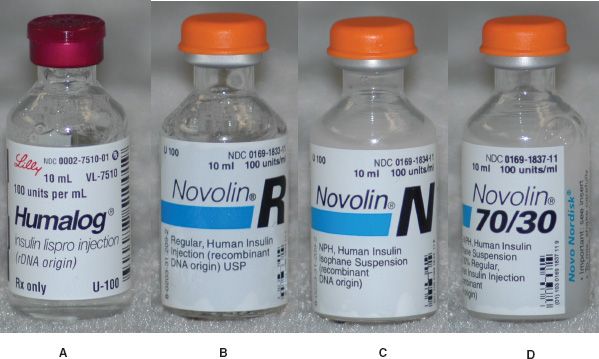
Figure 11.1Types of insulin preparations. (A) Humalog® is a rapid-acting insulin. (B) Novolin® R is a short-acting insulin. (C) Novolin® N is an intermediate-acting insulin. (D) Novolin® 70/30 is an intermediate- and short-acting mixture.
The most common insulin is U-100; U-100 means there are 100 units in 1 mL. It is commonly supplied in a 10-mL vial. U-500 is a more concentrated product containing 500 units in 1 mL, also supplied in a 10 mL-vial (Fig. 11.2).
Figure 11.2Lantus® is one brand of U-100 insulin.
 Heparin
Heparin
Heparin (Fig. 11.3), a product used to prevent clot formation and coagulation, is also ordered and manufactured in USP units. Heparin is available in a variety of strengths and ampuls or vial sizes, including products in 10 units/mL in 1-mL vials used for flushing heparin lock devices and 5000 to 10,000 units/mL in multiple-dose vials. Normal heparin doses are 20,000 to 40,000 units per day. Heparin is commonly ordered in units per hour and infused with an electronic infusion device.
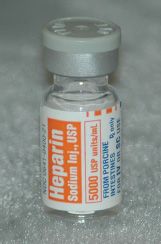
Figure 11.3Heparin prevents clot formation.
multiple-dose vial A product prepared with a preservative that can be safely used for more than one dose of the given mediation.
Critical Thinking 11.1