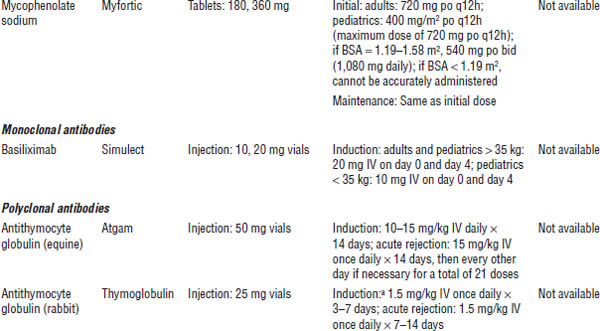
Table 24-1. Immunosuppressant Drugs
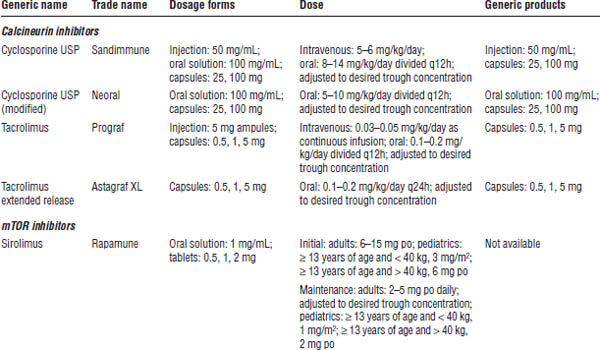

a. Medication is not FDA approved for induction.
Table 24-2. Drug Interactions Leading to Altered Exposure of CYP450 3A Isoenzyme Substrates
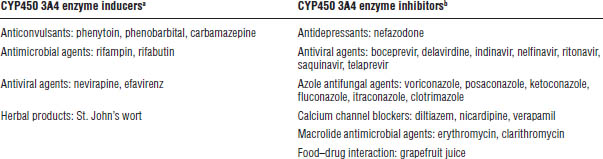
The table shows examples only. Numerous other interactions are associated with CYP450 3A4 substrates. See current journals or drug interaction texts for a more detailed list.
a. Inducers result in increased metabolism of substrates of the same system.
b. Inhibitors result in decreased metabolism of substrates of the same system.
Table 24-3. Drug Interactions Leading to Altered Exposure of Other Drugs by Cyclosporine
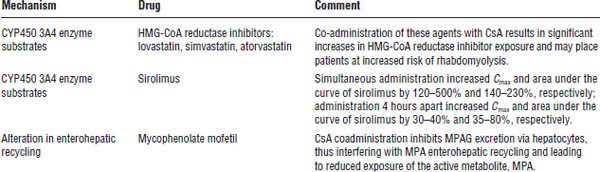
The table shows examples only. Numerous other interactions are associated with CYP450 3A4 substrates. See current journals or drug interaction texts for a more detailed list.
CsA, cyclosporine A; HMG-CoA, 3-hydroxy-3-methyglutaryl coenzyme A; MPA, mycophenolic acid; MPAG, phenolic glucuronide of MPA.
Adverse drug reactions
■ Central nervous system (CNS): Seizure, hallucinations, insomnia, tremor, paresthesias
■ Head, ears, eyes, nose, and throat (HEENT): Gingival hyperplasia
■ Cardiovascular (CV): Hypertension
■ Gastrointestinal (GI): Hepatotoxicity
■ Renal: Nephrotoxicity
■ Endocrine and metabolic: Hyperlipidemia, hyperuricemia, hyperkalemia, hypomagnesemia, new onset diabetes after transplant
■ Dermatologic: Hirsutism, hypertrichosis, acne
Patient instructions
■ Keep cyclosporine stored in its original container.
■ Take the prescribed dose twice daily with meals.
■ Keep timing of dosing consistent.
■ Make sure you do not take your morning medication on the day of therapeutic drug monitoring. After the monitoring is complete, take your morning medication immediately and then return to your original medication schedule.
■ Many medications interact with this medication. Do not take anything prescribed by another physician until you verify that there are no drug interactions.
Monitoring
■ C0 (trough): Goals depend on multifactorial risk assessment and assay type.
■ C2 (concentration 2 hours after dose): Goals depend on multifactorial risk assessment and assay type.
Pharmacokinetics
■ Cyclosporine USP: Highly lipoprotein bound
• Bioavailability: Significant intra- and interpatient variability
• Mean F = 30%, range 5% to 92%
• Elimination: Half-life = 19 hours; range 10–28 hours (increased with hepatic dysfunction)
■ Cyclosporine USP (modified): Highly lipoprotein bound
• Bioavailability: Improved and more consistent absorption (60–70% increased Cmax)
• Elimination: Half-life = 8 hours; range 5–18 hours (increased with hepatic dysfunction)
Tacrolimus
Mechanism of action
Tacrolimus inhibits translocation of the cytosolic subunit of NFAT, the promoter gene for IL-2, into the nucleus via its binding with the protein FKBP-12 and a calcium-calmodulin-calcineurin complex, thereby inhibiting transcription and synthesis of IL-2. Thus, it inhibits IL-2-mediated monoclonal T-cell proliferation and polyclonal T-cell activation.
Administration
■ Intravenous: Dilute in NS or D5W to a concentration between 0.004 and 0.02 mg/mL, and administer as a continuous infusion via a PVC (polyvinylchloride)–free container and tubing.
■ Oral: Administer immediate-release product in two equally divided doses orally every 12 hours consistently, with or without food. Administer extended-release product orally once every 24 hours consistently, with or without food.
Drug–drug interactions
Because tacrolimus is metabolized primarily via CYP450 3A isoenzymes, substances known to alter functionality of these enzymes will alter bioavailability and elimination of this drug (Table 24-2).
Drug–disease interactions
■ Diabetes mellitus: Administration worsens glycemic control in patients with preexisting diabetes.
■ Liver transplantation: The extended-release formulation has been associated with an increased risk of mortality in female liver transplant recipients.
■ Vaccinations: In general, immunosuppressants may affect efficacy of vaccinations. The use of live vaccines should be avoided.
Adverse drug reactions
■ CNS: Seizure, hallucinations, insomnia, tremor, depression, psychosis, anorexia
■ HEENT: Alopecia
■ CV: Hypertension, QT prolongation
■ GI: Hepatotoxicity
■ Renal: Nephrotoxicity
■ Endocrine and metabolic: Hyperlipidemia, hyperkalemia, hypercalcemia, hypomagnesemia, hypophosphatemia, new onset diabetes after transplant
■ Hematologic: Anemia
■ Other: Malignancies, infection
Patient instructions
■ Take the prescribed dose at a consistent time once or twice daily (extended-release or immediate-release formulations), with or without food, but always in the same way to maintain consistency.
■ Make sure you do not take your morning medication on the day of therapeutic drug monitoring. After the monitoring is complete, take your morning medication immediately and then return to your original medication schedule.
■ Many medications interact with this medication. Do not take anything prescribed by another physician until you verify that there are no drug interactions.
Monitoring
Monitor C0 (trough). Goals depend on multifactorial risk assessment (in general, 5–15 ng/mL).
Pharmacokinetics
■ Highly protein bound
■ Bioavailability: F = 14–32%
■ Elimination: Half-life = 8 hours; range 6–11 hours (increased with hepatic dysfunction); ~ 37 hours for extended-release formulation
mTOR Inhibitors
Everolimus
Mechanism of action
Everolimus binds to FKBP-12 to form a complex that binds and inhibits activation of its target protein, mTOR, a kinase that is critical in IL-2-mediated cell-cycle progression.
Administration
■ To limit variability, administer consistently with or without food.
■ With tablets, administer dose po bid.
Drug–drug interactions
Because everolimus is metabolized primarily via CYP450 3A isoenzymes, substances known to alter functionality of these enzymes will alter bioavailability and elimination of this drug (Table 24-2).
Additionally, the pharmacokinetic profile of everolimus is significantly altered by concomitant cyclosporine (Table 24-3).
Drug–disease interactions
■ Kidney transplantation: Everolimus is associated with arterial and venous thrombosis of the kidney allograft and may cause proteinuria.
■ Edema: Everolimus has been associated with peripheral edema and pleural and pericardial effusions.
■ Infertility: Everolimus has been associated with male infertility.
■ Hyperlipidemia: Everolimus increases triglycerides and cholesterol.
■ Vaccinations: In general, immunosuppressants may affect efficacy of vaccinations. The use of live vaccines should be avoided.
Adverse drug reactions
■ CNS: Fatigue, headache
■ HEENT: Oral ulcers
■ GI: Anorexia, constipation, diarrhea, nausea
■ Renal: Synergistic nephrotoxicity with calcineurin inhibitors, proteinuria
■ Endocrine and metabolic: Hyperlipidemia, hypertension, hyperkalemia
■ Hematologic: Anemia, leukopenia, thrombocytopenia, pancytopenia, thrombosis
■ Other: Lymphocele, pneumonitis
Patient instructions
■ Take the prescribed dose at a consistent time twice daily, with or without food.
■ Make sure you do not take your medication before therapeutic drug monitoring.
■ Many medications interact with this medication. Do not take anything prescribed by another physician until you verify that there are no drug interactions.
Monitoring
Monitor C0 (trough). Goal depends on multifactorial risk assessment and assay type (in general, 3–8 ng/mL).
Pharmacokinetics
■ Bioavailability: F = 30%
■ Elimination: Half-life = 30 hours (increased with hepatic dysfunction)
Sirolimus
Mechanism of action
Sirolimus binds to FKBP-12 to form a complex that binds and inhibits activation of its target protein, mTOR, a kinase that is critical in IL-2-mediated cell-cycle progression.
Administration
■ To limit variability, administer consistently with or without food.
■ With tablets, administer daily dose po once a day.
■ With oral solution, dilute the dose in 2 oz of water or orange juice, stir vigorously, and drink at once. Then refill container with 4 oz of the chosen fluid, stir vigorously, and drink.
Drug–drug interactions
Because sirolimus is metabolized primarily via CYP450 3A isoenzymes, substances known to alter functionality of these enzymes will alter bioavailability and elimination of this drug (Table 24-2).
Additionally, the pharmacokinetic profile of sirolimus is significantly altered by concomitant cyclosporine (Table 24-3).
Drug–disease interactions
■ Edema: Sirolimus has been associated with peripheral edema and pleural and pericardial effusions.
■ Hyperlipidemia: Sirolimus increases levels of triglycerides and cholesterol.
■ Kidney transplantation: Sirolimus may delay recovery of graft function post-transplant and is associated with increased urinary protein excretion after conversion from calcineurin inhibitors.
■ Liver transplantation: Sirolimus is associated with increased incidence of mortality, graft loss, and hepatic artery thrombosis in de novo liver transplant recipients.
■ Lung transplantation: There have been cases of fatal bronchial anastomotic dehiscence in de novo lung transplant recipients.
■ Vaccinations: In general, immunosuppressants may affect efficacy of vaccinations. The use of live vaccines should be avoided.
Adverse drug reactions
■ CNS: Anorexia
■ HEENT: Oral ulcers
■ GI: Diarrhea, esophagitis, gastritis, gastroenteritis, hepatotoxicity, hepatic artery thrombosis in de novo liver transplant recipients
■ Renal: Synergistic nephrotoxicity with calcineurin inhibitors, proteinuria
■ Endocrine and metabolic: Hyperlipidemia, hypertension, hyperkalemia
■ Dermatologic: Rash, acne
■ Hematologic: Leukopenia, thrombocytopenia, pancytopenia, thrombosis
■ Other: Arthralgia, lymphocele, pneumonitis, bronchial anastomotic dehiscence in de novo lung transplant recipients
Patient instructions
■ Take the prescribed dose at a consistent time once daily, with or without food, but in the same way to maintain consistency.
■ Make sure you do not take your morning medication on the day of therapeutic drug monitoring. After the monitoring is complete, take your morning medication immediately and then return to your original medication schedule.
■ Many medications interact with this medication. Do not take anything prescribed by another physician until you verify that there are no drug interactions.
Monitoring
Monitor C0 (trough). Goal depends on multifactorial risk assessment and assay type (in general, 5–15 ng/mL).
Pharmacokinetics
■ Bioavailability: F = 14%
■ Elimination: Half-life = 57–63 hours (increased with hepatic dysfunction)
Selective T-Cell Co-stimulation Blocker
Belatacept
Mechanism of action
Belatacept binds to co-stimulation proteins CD80 and CD86 on the APC, to prevent binding to the T-cell proteins (i.e., CD28) necessary for activation of the T-cell.
Administration
Intravenous: Reconstitute contents with 10.5 mL of sterile water for injection, NS, or D5W. Calculate total volume of reconstituted solution necessary for prescribed dose. The reconstituted solution is diluted with NS or D5W to a final concentration of 2–10 mg/mL. Administer peripherally or centrally over 30 minutes with a 0.1–1.2 µm low-protein-binding filter. Refer to Table 24-1 for dosing schedule.
Drug–drug interactions
No clinically significant interactions have been reported.
Drug–disease interactions
■ Liver transplantation: In a clinical trial with more frequent administration than noted in Table 24-1, belatacept was associated with increased graft loss and mortality.
■ Post-transplant lymphoproliferative disease (PTLD): Belatacept is associated with an increased risk of PTLD involving the central nervous system, and thus is avoided in patients that are Epstein-Barr virus seronegative or with unknown status.
■ Tuberculosis: An increased incidence of tuberculosis has been reported with belatacept; thus patients should be evaluated for latent tuberculosis infection before administering belatacept.
■ Vaccinations: In general, immunosuppressants may affect efficacy of vaccinations. The use of live vaccines should be avoided.
Adverse drug reactions
■ CNS: Headache, insomnia, anxiety, PTLD
■ CV: Hypertension
■ GI: Diarrhea, constipation, nausea, vomiting, abdominal pain
■ Hematologic: Anemia, leukopenia
■ Renal: Hematuria, proteinuria, dysuria
■ Endocrine and metabolic: Hypocalcemia, hypo- or hyperkalemia, hypophosphatemia, dyslipidemia, hyperglycemia
■ Dermatologic: Acne
■ Other: Urinary tract infection, peripheral edema, upper respiratory infection, progressive multifocal leukoencephalopathy, polyomavirus nephropathy
Patient instructions
Report any adverse drug reactions to your health care provider immediately.
Monitoring
Patients should be monitored closely for signs and symptoms of infection or PTLD (i.e., behavioral changes).
Pharmacokinetics
■ Dosing is based on total body weight.
■ Linear pharmacokinetics: First-order elimination (standard doses in Table 24-1)
■ Elimination: Half-life = ~11 days
Antiproliferative Agents
Azathioprine
Mechanism of action
Azathioprine is a purine analogue pro-drug, which is cleaved to 6-mercaptopurine; 6-mercaptopurine is activated intracellularly to several active metabolites, which can be incorporated directly into DNA (deoxyribonucleic acid) as thiopurine as well as interfere with the RNA (ribonucleic acid) and DNA biosynthesis directly and via feedback inhibition.
Administration
■ Intravenous: Dilute dose in NS or D5W, and administer IV infusion over 5–60 minutes.
■ Oral: Administer daily dose po once a day.
Drug–drug interactions
Xanthine oxidase is responsible for the elimination of the active metabolites of azathioprine. Concomitant use of allopurinol with azathioprine results in significantly increased azathioprine-induced toxicity. Reduce dose of azathioprine by 65–75%.
Drug–disease interactions
■ Renal insufficiency: Bioavailability is significantly reduced in uremic patients.
■ Vaccinations: In general, immunosuppressants may affect efficacy of vaccinations. The use of live vaccines should be avoided.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree