(1)
Department of Pathology, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
Keywords
LiposarcomaAngiosarcomaHemangiomaEpithelioid hemangioendotheliomaFibrosarcomaSolitary fibrous tumorMalignant fibrous histiocytomaLeiomyosarcomaRhabdomyosarcomaChondrosarcomaChordomaOsteosarcomaSynovial sarcomaAlveolar soft part sarcomaMalignant rhabdoid tumorMesenchymal tumors of the mediastinum are relatively rare, yet virtually all types of mesenchymal neoplasms have been described in this location (Table 5.1).
Table 5.1
Mesenchymal tumors of the mediastinum
Category | Tumor type |
|---|---|
Vascular tumors | Hemangioma |
Lymphangioma | |
Epithelioid hemangioendothelioma | |
Angiosarcoma | |
Fibroblastic/fibrohistiocytic tumors | Solitary fibrous tumor |
Malignant solitary fibrous tumor | |
Malignant fibrous histiocytoma (undifferentiated sarcoma) | |
Myogenic tumors | Leiomyoma |
Leiomyosarcoma | |
Rhabdomyosarcoma | |
Malignant “triton” tumor | |
Lipomatous tumors | Lipoma |
Atypical lipomatous tumor/well-differentiated liposarcoma | |
Dedifferentiated liposarcoma | |
Myxoid/round cell liposarcoma | |
Pleomorphic liposarcoma | |
Bone and cartilaginous tumors | Chondrosarcoma |
Chordoma | |
Extraskeletal mesenchymal chondrosarcoma | |
Myxoid chondrosarcoma | |
Extraskeletal osteosarcoma | |
Tumors of unknown etiology | Malignant rhabdoid tumor |
Synovial sarcoma | |
Alveolar soft part sarcoma |
5.1 Vascular Tumors
The majority of tumors originating from vascular endothelium in the mediastinum are benign conditions that most likely represent malformative or congenital processes occurring during childhood or adolescence (Figs. 5.1, 5.2, 5.3, 5.4, 5.5, 5.6, 5.7, 5.8, 5.9, 5.10, 5.11, 5.12, 5.13, 5.14, 5.15, 5.16, 5.17, 5.18, 5.19, 5.20, 5.21, 5.22, 5.23, 5.24, 5.25, 5.26, 5.27, 5.28, 5.29, 5.30, 5.31, 5.32, 5.33, 5.34, 5.35, 5.36, 5.37, 5.38, 5.39, 5.40, 5.41, 5.42, and 5.43). Benign, low-grade, and malignant vascular neoplasms are extremely rare in the mediastinum and tend to occur more often in the pediatric population. Paradoxically, one of the rarest forms of low-grade malignant vascular neoplasm, epithelioid hemangioendothelioma (Figs. 5.7, 5.8, 5.9, 5.10, 5.11, 5.12, 5.13, 5.14, 5.15, 5.16, 5.17, 5.18, 5.19, 5.20, 5.21, 5.22, 5.23, 5.24, 5.25, 5.26, 5.27, 5.28, 5.29, 5.30, 5.31, 5.32, 5.33, 5.34, 5.35, 5.36, and 5.37), represents the vascular malignancy most commonly encountered in the mediastinum in adults.
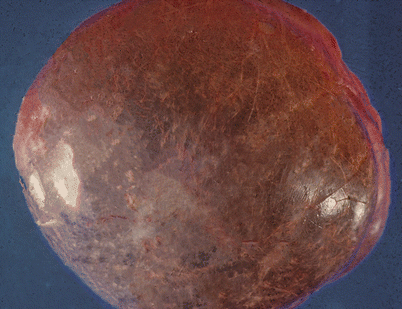
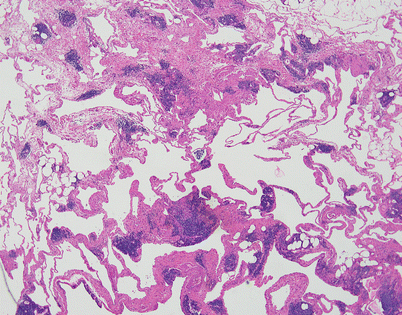
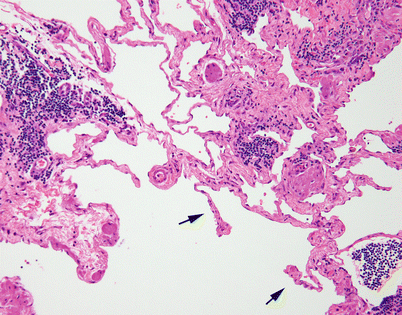
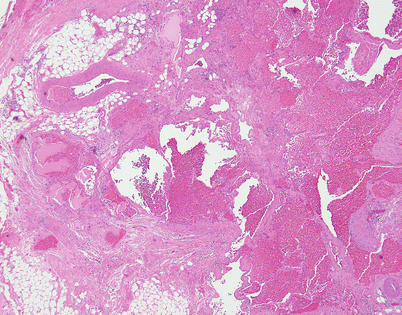
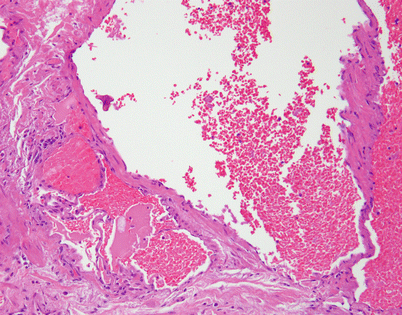
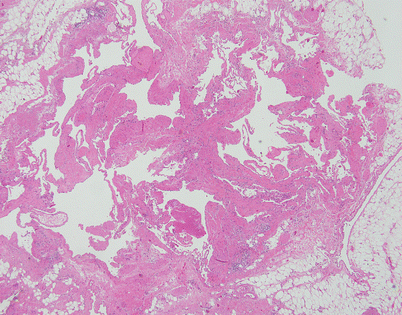
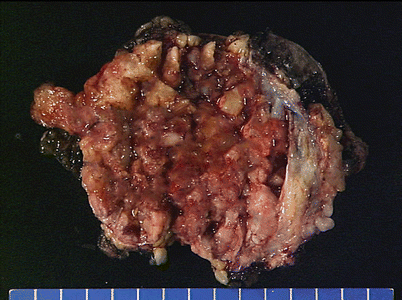
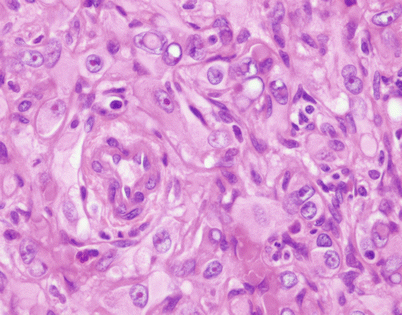
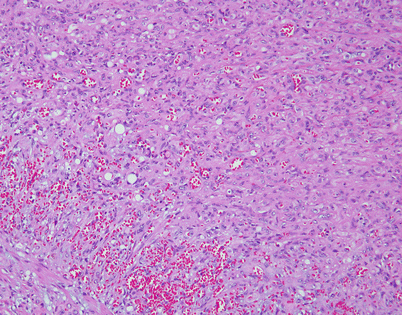
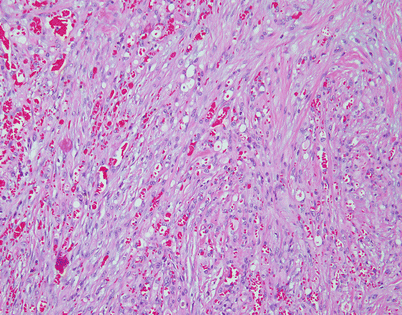
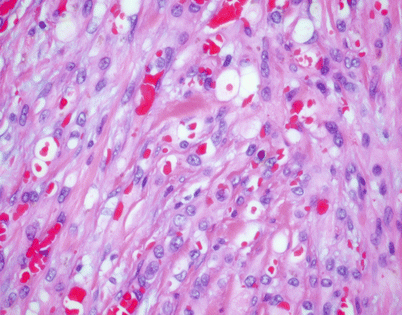
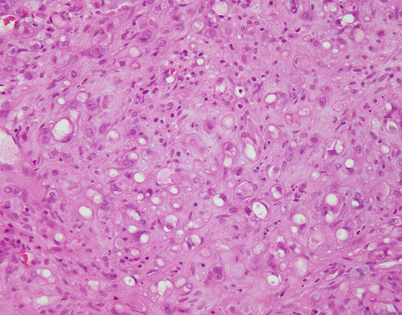
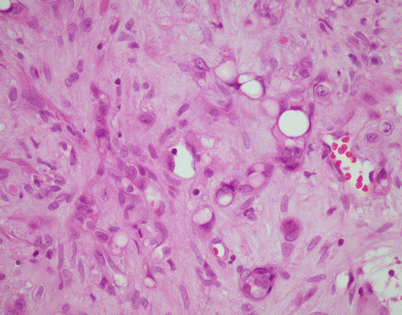
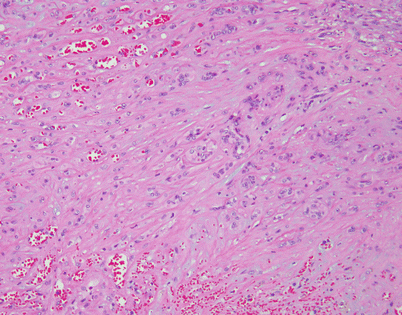
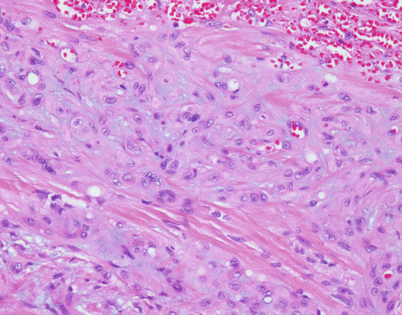
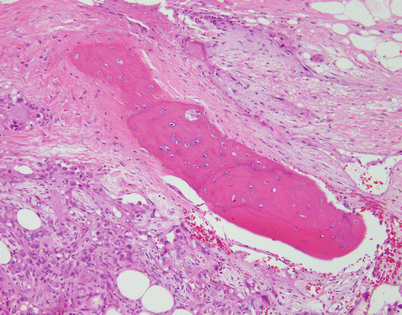
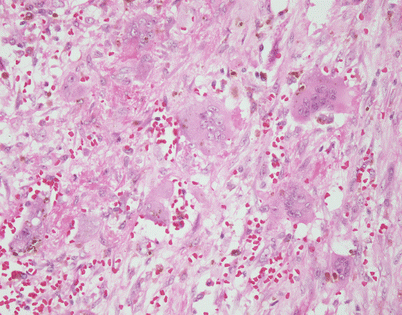
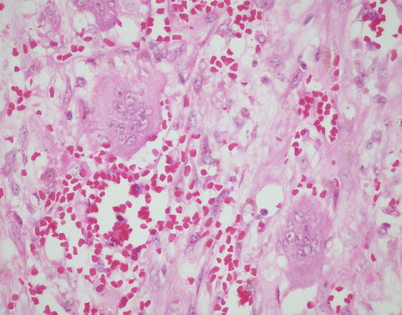
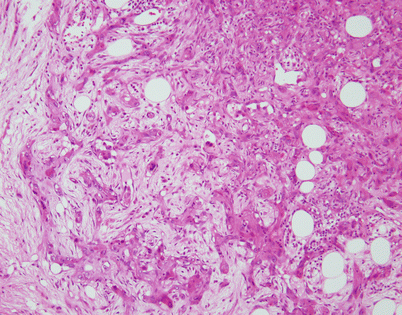
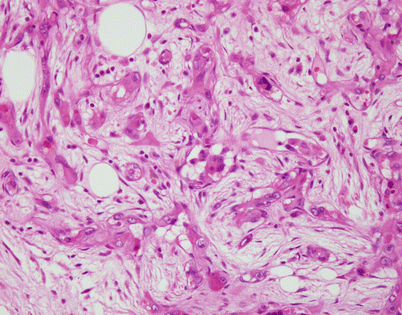
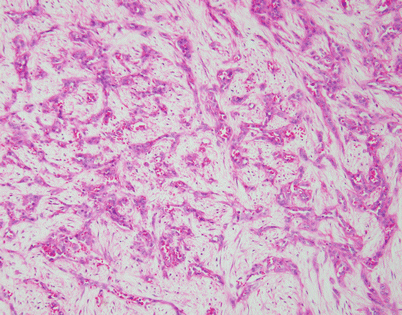
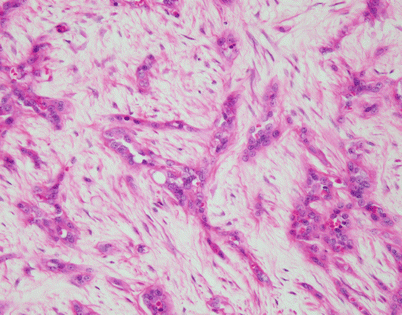
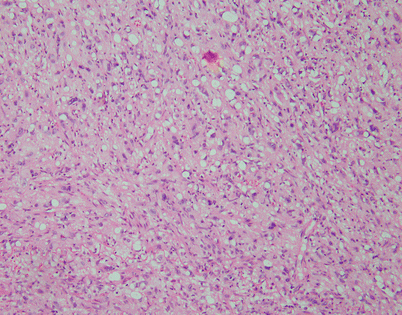
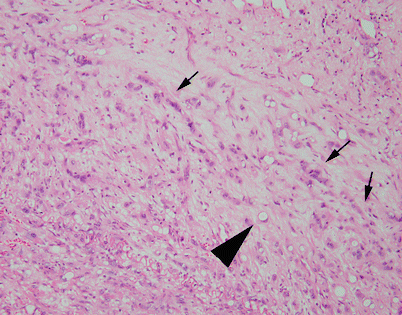
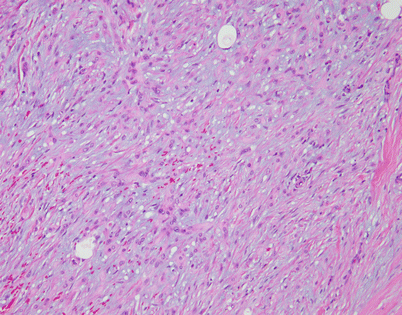
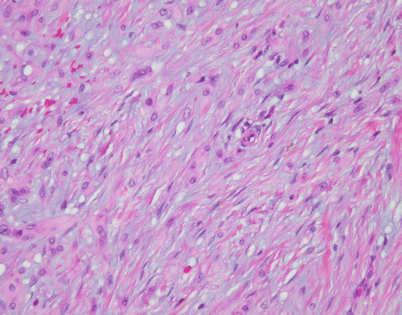
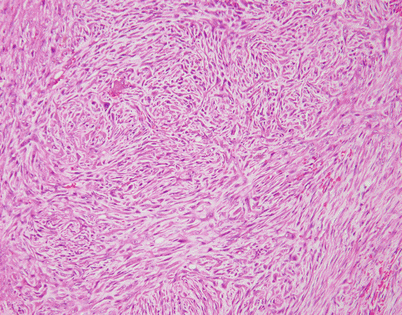
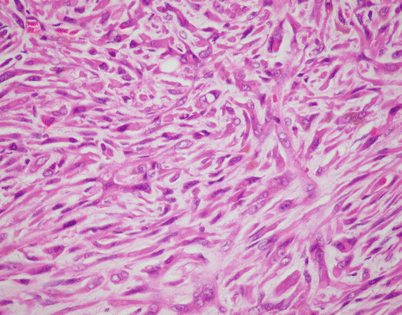
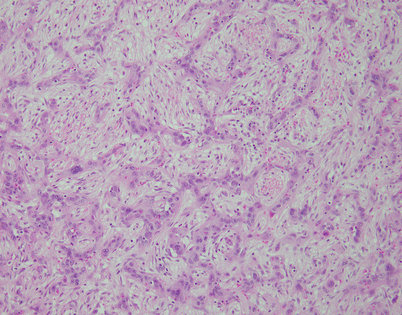
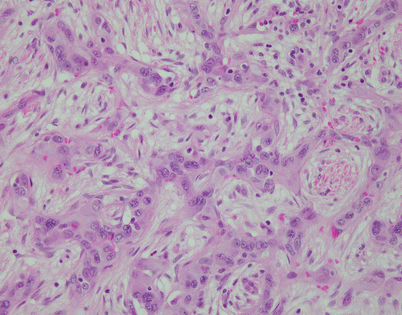
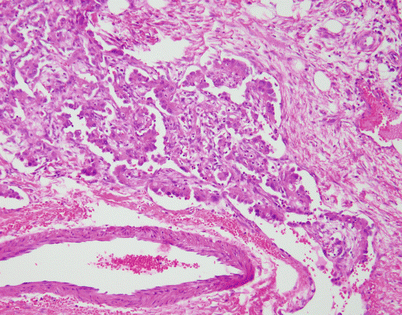
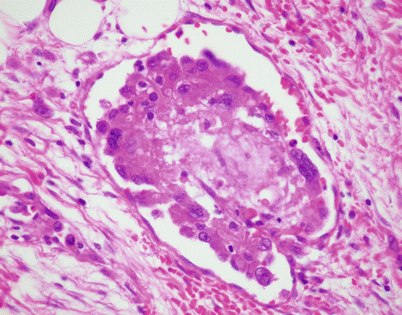
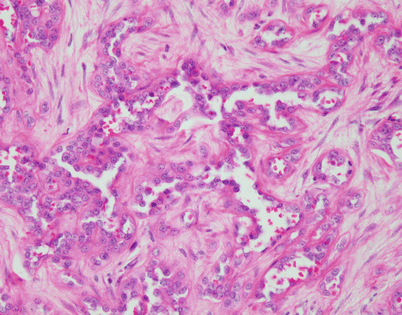
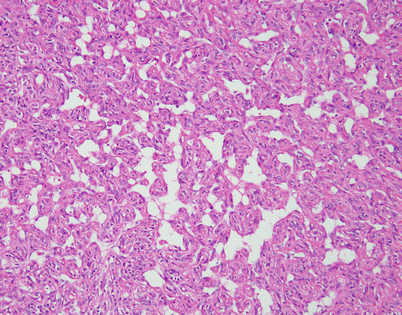
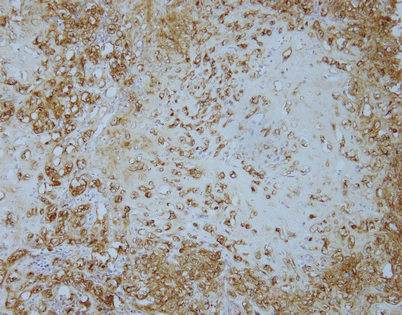
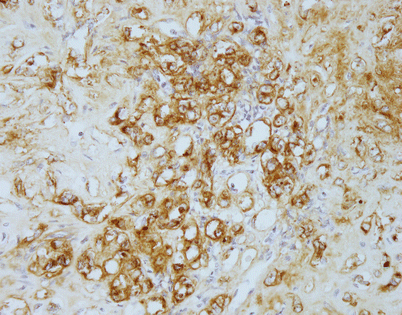
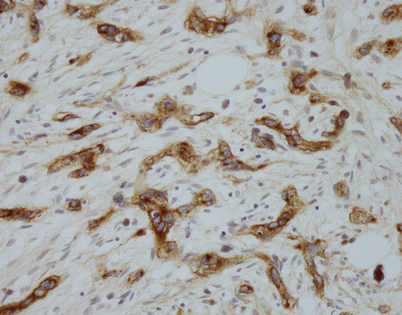
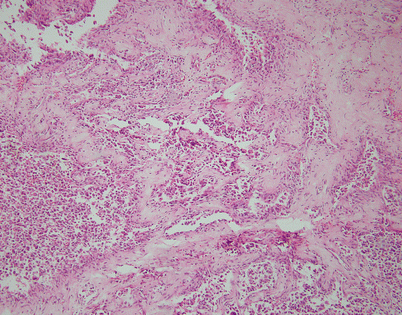
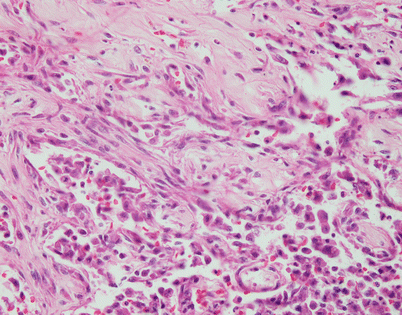
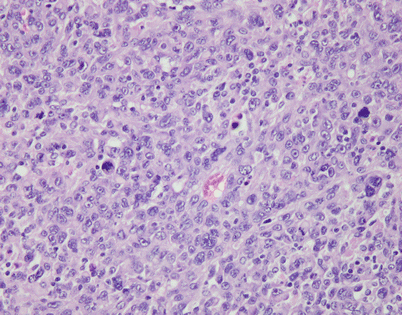
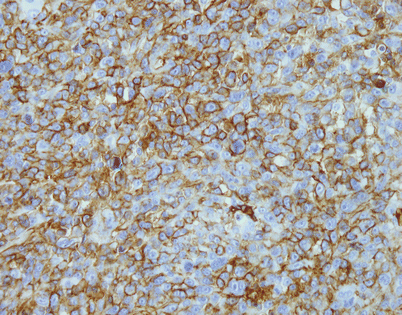
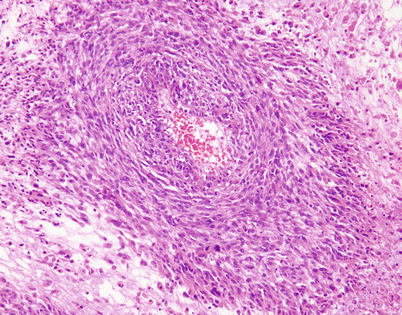
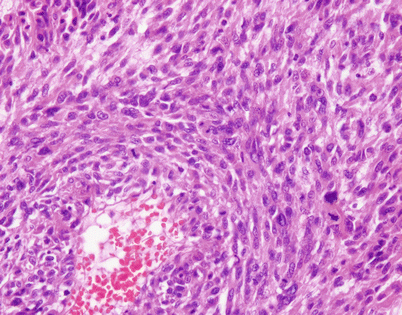

Fig. 5.1
Gross appearance of cystic lymphangioma (cystic hygroma) of the mediastinum in a child shows a smooth and shiny outer surface distended by fluid

Fig. 5.2
Cystic lymphangiomas are composed of multiple, cystically dilated lymphatics that often contain dense, lymphoid aggregates in their walls. The dilated empty spaces may contain occasional small lymphocytes but are devoid of red blood cells

Fig. 5.3
On higher magnification, cystic lymphangiomas will disclose small, rudimentary valves (arrows) in charge of regulating the lymph flow

Fig. 5.4
Mediastinal hemangiomas are most commonly of the cavernous type and are characterized by multiple dilated vessels distended with red blood cells

Fig. 5.5
At higher magnification, the mediastinal hemangioma shows thin-walled vessels lined by a single layer of endothelial cells and containing red blood cells in their lumen

Fig. 5.6
Mediastinal hemangiomas are most likely hamartomatous malformations that often contain other components admixed with the vascular elements, such as smooth muscle, fat, and cartilage. This example shows a well-developed smooth muscle component surrounding the vessels, along with mature stromal fat

Fig. 5.7
Epithelioid hemangioendothelioma is the most common malignant vascular neoplasm of the mediastinum, where they are characterized by relatively low-grade malignant behavior and are associated with a very good survival rate following complete surgical excision. The cut surface often shows a fleshy, multinodular appearance with congestion and foci of hemorrhage. Gritty foci of calcifications are also often noted

Fig. 5.8
Histologically, epithelioid hemangioendothelioma is characterized by a proliferation of large epithelioid cells with enlarged nuclei and abundant cytoplasm that can closely resemble an epithelial malignancy. The nuclei show dispersed chromatin and prominent eosinophilic nucleoli; intranuclear cytoplasmic pseudoinclusions can be seen. Despite the atypical appearance of the cells, mitoses are inconspicuous

Fig. 5.9
Epithelioid hemangioendothelioma vary greatly in growth patterns and cytologic appearance. The most common growth pattern is a solid pattern composed of sheets of relatively monotonous epithelioid tumor cells

Fig. 5.10
A distinguishing and characteristic feature of epithelioid hemangioendothelioma is the presence of multiple, scattered, single cytoplasmic vacuoles associated with the tumor cells

Fig. 5.11
On higher magnification, the small cytoplasmic vacuoles are seen to contain single red blood cells within their lumen. It has been postulated that these cells may indicate an attempt by the tumor cells to create abortive vascular lumens

Fig. 5.12
Another example of mediastinal epithelioid hemangioendothelioma shows a sheet of large epithelioid cells dotted by numerous small, empty vacuoles that are quite conspicuous on scanning magnification

Fig. 5.13
Higher magnification from Fig. 5.12 shows large, epithelioid cells with small cytoplasmic vacuoles. In some of the cells, the vacuoles compress the nuclei to the periphery, creating a signet-ring cell appearance

Fig. 5.14
Stromal changes are a common feature of epithelioid hemangioendothelioma of the mediastinum, including stromal hyalinization and deposition of myxoid matrix. This example shows an area with extensive hyalinization and collagenization of the stroma

Fig. 5.15
Another area from the same tumor shows prominent deposits of myxoid matrix that closely resemble the appearance of a cartilaginous matrix

Fig. 5.16
Another unusual feature that is more commonly encountered in an epithelioid hemangioendothelioma in a mediastinal location is the formation of metaplastic bone. The bony spicules can be small and randomly scattered, or they can become quite prominent and easily identified on chest imaging studies

Fig. 5.17
A somewhat related phenomenon observed in mediastinal epithelioid hemangioendothelioma is the presence of clusters of osteoclastic, multinucleated giant cells in the stroma. It has been postulated that this phenomenon is related to focal hemorrhage and that the cells appear as a result of chemotactic factors in response to the breakdown of red blood cells

Fig. 5.18
Higher magnification from Fig. 5.17 shows two large, osteoclast-type multinucleated cells with numerous small nuclei. The cells are surrounded by abundant extravasated red blood cells

Fig. 5.19
A less frequently encountered pattern of growth in epithelioid hemangioendothelioma is characterized by anastomosing cords of tumor cells that are separated by abundant fibrous stroma, further enhancing the similarity to an epithelial malignant neoplasm

Fig. 5.20
Higher magnification from Fig. 5.19 shows cords of epithelioid tumor cells embedded in fibrous stroma, simulating a desmoplastic reaction by tumor

Fig. 5.21
Another example of epithelioid hemangioendothelioma of the mediastinum shows a pseudoinfiltrative growth pattern composed of short, irregular cords of large, epithelioid tumor cells

Fig. 5.22
Higher magnification shows irregular cords of epithelioid cells, some of which appear to be forming imperfect or abortive lumens, closely resembling a poorly differentiated carcinoma

Fig. 5.23
The combination of abundant cytoplasmic vacuoles in the tumor cells with a cord-like growth pattern can also enhance the similarity with a metastatic carcinoma and raise the possibility of a signet-ring cell carcinoma. Numerous scattered vacuoles containing nuclei that have been displaced to the periphery are seen in this field

Fig. 5.24
Higher magnification from the same case as Fig. 5.23 shows discrete cords of atypical tumor cells (small arrows) embedded in loose connective tissue. A cell with a cytoplasmic vacuole (large arrowhead) closely simulates a signet-ring cell

Fig. 5.25
Another unusual appearance of epithelioid hemangioendothelioma is caused by spindling of the tumor cells. The spindling may be a result of artifactual compression of tumor cells by stromal overgrowth

Fig. 5.26
Higher magnification of epithelioid hemangioendothelioma with spindling of tumor cells shows compressed, elongated nuclei with tapered ends surrounded by abundant stromal collagen. Such cases can be confused with a variety of spindle-cell sarcomas

Fig. 5.27
Another example of epithelioid hemangioendothelioma of the mediastinum with a spindle-cell growth pattern. This case shows a clearly fascicular pattern composed of spindle cells with focal storiform pattern (top left)

Fig. 5.28
Higher magnification of the example of epithelioid hemangioendothelioma in Fig. 5.27 shows cells with elongated, hyperchromatic nuclei surrounded by abundant eosinophilic cytoplasm

Fig. 5.29
This example of epithelioid hemangioendothelioma of the mediastinum shows a peculiar retiform pattern of growth, with anastomosing trabeculae of tumor cells circumscribing islands of loose connective tissue

Fig. 5.30
Higher magnification from Fig. 5.29 shows anastomosing trabeculae of tumor cells with abundant epithelioid cytoplasm, resembling an epithelial malignancy

Fig. 5.31
Another unusual feature seen in mediastinal epithelioid hemangioendothelioma is intravascular growth of tumor cells. The tumor grows as polypoid or glomeruloid clusters of cells filling the lumen of dilated vessels

Fig. 5.32
Higher magnification of epithelioid hemangioendothelioma of the mediastinum with intravascular tumor growth shows a small vessel with the lumen filled by a polypoid collection of large epithelioid cells with abundant eosinophilic cytoplasm

Fig. 5.33
Vessel-like growth pattern in an epithelioid hemangioendothelioma of the mediastinum. Large epithelioid tumor cells are seen lining anastomosing vascular spaces containing scattered intraluminal red blood cells. There appears to be a continuous spectrum between epithelioid hemangioendothelioma and epithelioid angiosarcoma; in some cases, areas may be indistinguishable from epithelioid angiosarcoma

Fig. 5.34
Another example of epithelioid hemangioendothelioma with vessel-like spaces resulting in a striking hemangiopericytoma-like pattern of growth. Multiple small vessels with branching, patent lumens are seen surrounded by the large epithelioid cells

Fig. 5.35
Epithelioid hemangioendothelioma is easily diagnosed with the use of immunohistochemical markers. Stains for factor VIII, Ulex europaeus lectin, CD34, and CD31 will stain the tumor cells. The image shows positivity of the tumor cells for CD31 in a case of mediastinal epithelioid hemangioendothelioma

Fig. 5.36
Unlike angiosarcomas, which show inconsistent positivity for factor VIII (von Willebrand factor), epithelioid hemangioendotheliomas are extremely sensitive for this stain. Almost all show strong positivity for this antigen, as depicted in this image. It is important to consider the possibility of epithelioid hemangioendothelioma in the diagnosis and include vascular markers in the panel of stains ordered, because many of these tumors may be also positive for cytokeratins; ordering only cytokeratin may lead to an incorrect diagnosis

Fig. 5.37
Positivity for factor VIII-related antigen (von Willebrand factor) is seen in this example of mediastinal epithelioid hemangioendothelioma with a trabecular growth pattern. The tumor closely resembled a metastatic carcinoma on hematoxylin and eosin (H&E)-stained sections, but positive staining for factor VIII and CD31 helped establish the correct diagnosis

Fig. 5.38
Angiosarcoma is a very rare tumor in the mediastinum. The tumors present as large, bulky, and hemorrhagic masses and may show a spectrum that ranges from very well-differentiated tumors composed of anastomosing vascular channels lined by atypical endothelial cells to poorly differentiated lesions that can resemble other high-grade sarcomas. The image shows an example of a well-differentiated angiosarcoma showing well-developed vascular channels

Fig. 5.39
Higher magnification from the tumor in Fig. 5.38 shows anastomosing vascular channels lined by atypical endothelial cells dissecting through the stromal collagen

Fig. 5.40
The epithelioid variant of angiosarcoma is characterized by sheets of large epithelioid cells with marked cytologic atypia. Notice the enlarged nuclei with prominent nucleoli and scattered mitotic figures. Diagnosis of these tumors can be a challenge if angiosarcoma is not included in the differential diagnosis. Use of appropriate immunohistochemical stains can be of assistance in arriving at the correct diagnosis

Fig. 5.41
Immunohistochemical staining for CD31 shows strong membranous and cytoplasmic positivity in the tumor cells of epithelioid angiosarcoma of the mediastinum. Borderline cases between epithelioid hemangioendothelioma and epithelioid angiosarcoma can be observed. A novel molecular alteration—rearrangement of the CAMTA1 gene leading to the fusion of CAMTA1/WWTR1—has been recently identified in high-grade epithelioid hemangioendothelioma but is not present in epithelioid angiosarcoma. Use of fluorescence in situ hybridization (FISH) probes may be helpful in separating the two conditions

Fig. 5.42
Angiosarcomas of the mediastinum can also be composed of atypical spindle cells and can resemble other types of soft tissue sarcomas. A search for more conventional areas demonstrating anastomosing vascular channels will be helpful in the differential diagnosis

Fig. 5.43
Higher magnification of a mediastinal angiosarcoma with spindle-cell morphology shows atypical spindle cells growing in fascicles with numerous mitotic figures. The tumor cells were positive for CD31 and other vascular-associated markers, such as FLI1, WT1, and ERG
5.2 Fibroblastic and “Fibrohistiocytic” Tumors
Tumors composed of fibroblastic cells represent the most common type of mesenchymal neoplasms in the mediastinum (Figs. 5.44, 5.45, 5.46, 5.47, 5.48, 5.49, 5.50, 5.51, 5.52, 5.53, 5.54, 5.55, 5.56, 5.57, 5.58, 5.59, 5.60, 5.61, 5.62, 5.63, 5.64, 5.65, 5.66, 5.67, 5.68, 5.69, 5.70, 5.71, 5.72, 5.73, 5.74, 5.75, 5.76, 5.77, 5.78, 5.79, 5.80, 5.81, 5.82, 5.83, 5.84, 5.85, 5.86, 5.87, and 5.88). Most are solitary fibrous tumors (SFTs), a mesenchymal neoplasm that is composed of dendritic fibroblastic cells that show a peculiar CD34/bcl2+ immunophenotype and are believe to represent specialized fibroblasts involved in antigen presentation in soft tissues. The vast majority of SFTs are benign; the malignant counterpart of such tumors has been variously termed malignant SFT or “dedifferentiated” SFT but could just as easily be simply termed fibrosarcoma. To establish a diagnosis of malignant SFT, most authors would require demonstration of a preexisting area of conventional SFT. Cases in which areas characteristic of conventional, benign SFT are not demonstrable have been variously assigned to the category of undifferentiated sarcomas, called “malignant fibrous histiocytoma” in the old literature.
A number of immunohistochemical markers have been used in recent years to identify the better-differentiated tumors (SFTs), including CD34, bcl-2, and the recently developed STAT6 antibody, which identifies a specific translocation that has been consistently demonstrated in these tumors. The undifferentiated tumors, on the other hand, largely represent a diagnosis of exclusion; there are no markers that permit assigning them to any specific category. It should be emphasized that the main differential diagnosis of malignant (and some benign) SFTs in the mediastinum includes dedifferentiated liposarcomas and monophasic synovial sarcomas. The latter are easily distinguishable from SFTs by their reactivity for cytokeratin and epithelial membrane antigen (EMA) and by the distinctive X;18 translocation; the former may be more difficult to distinguish from SFTs because they can share STAT6 and CD34 positivity and will require identification of areas of conventional liposarcoma and MDM2 positivity for differential diagnosis.
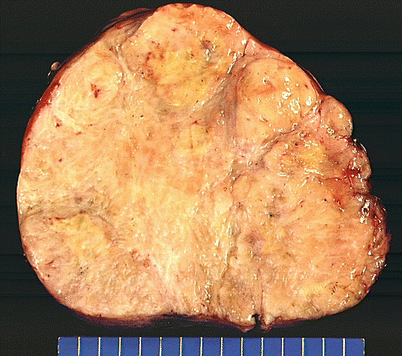
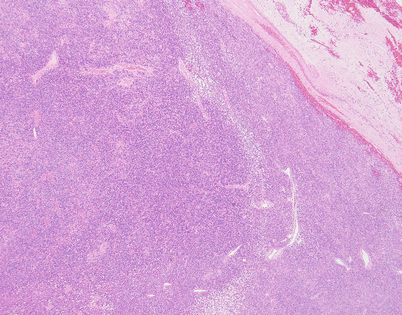
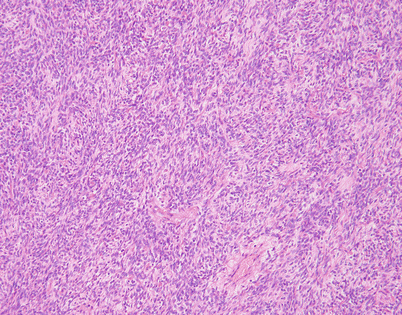
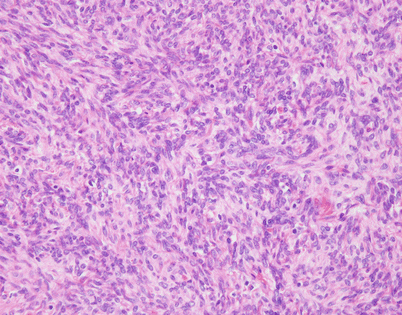
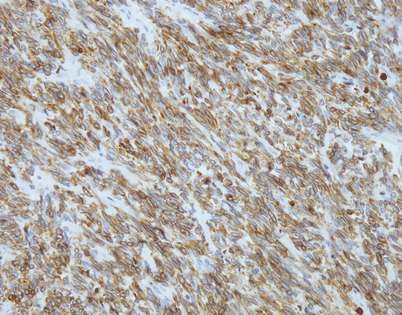
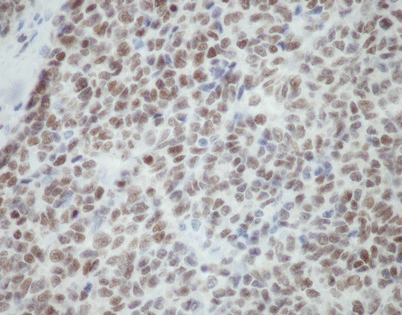
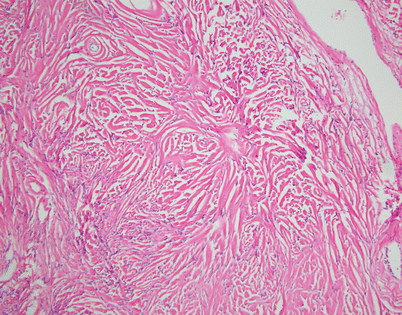
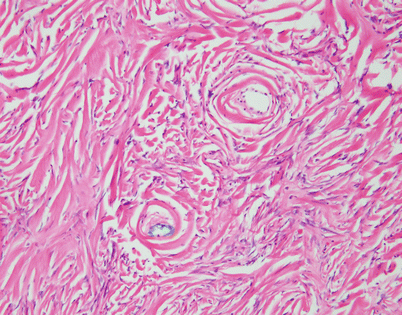
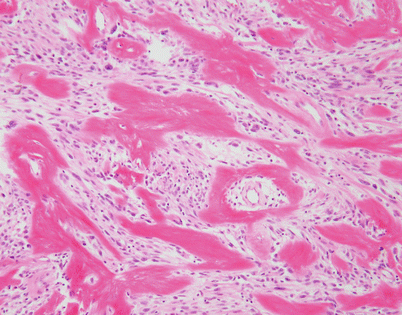
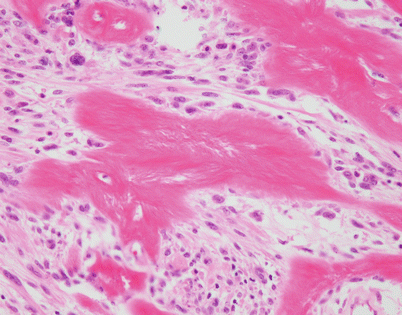
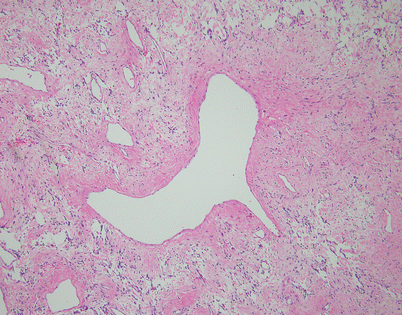
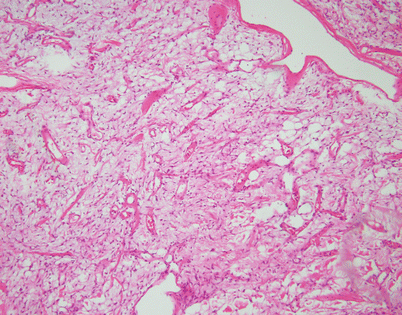
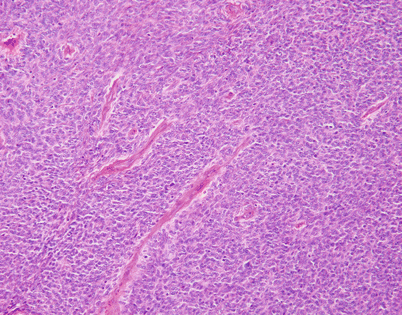
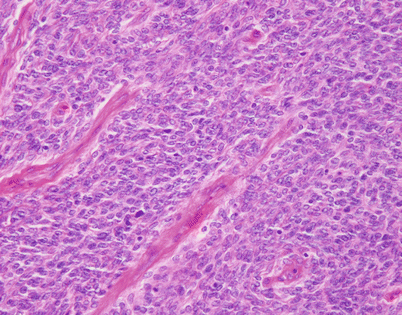
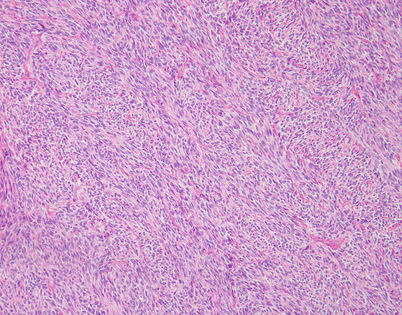
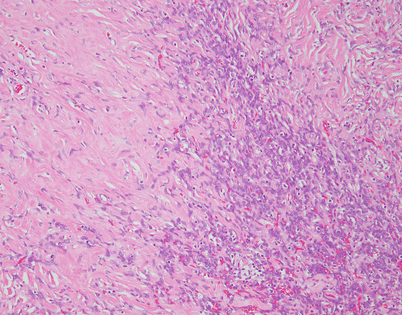
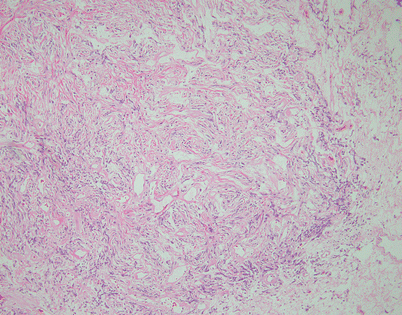
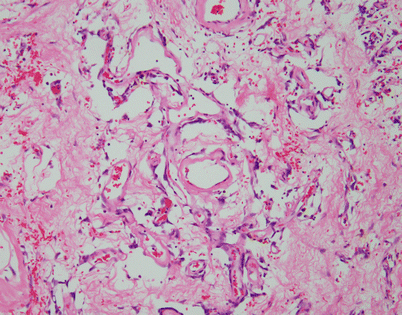
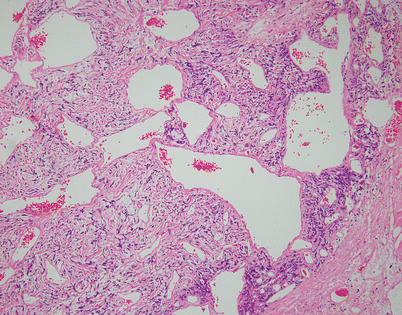
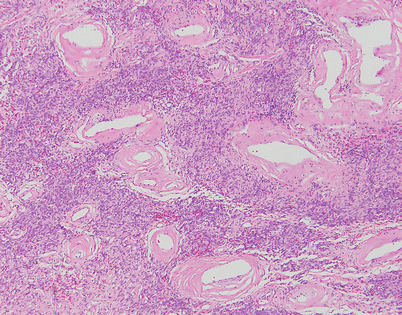
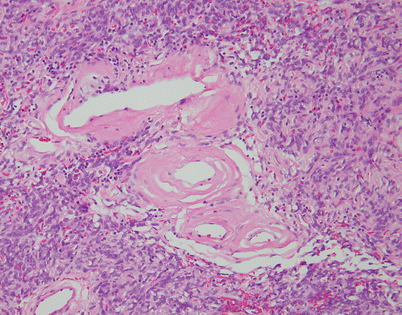
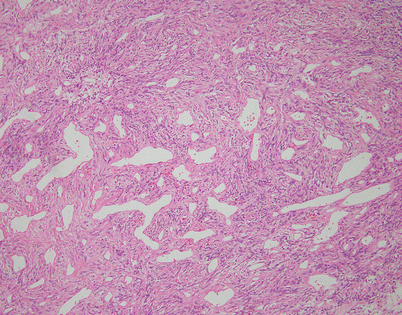

Fig. 5.44
Solitary fibrous tumors (SFTs) are usually fleshy and well-circumscribed masses showing a lobulated, tan-white cut surface. In the mediastinum, however, the tumors more often appear to be invasive and poorly circumscribed, and they tend to attain a larger size than their pleural counterparts

Fig. 5.45
SFTs are characterized by the striking variety of growth patterns they can display. The most common form is characterized by a bland-appearing, fibroblast-like spindle-cell proliferation with varying degrees of stromal collagenization. On scanning magnification, an SFT usually shows sharp circumscription from the surrounding tissues and is composed of a dense spindle-cell population with vessels showing open lumens scattered throughout

Fig. 5.46
On higher magnification, an SFT shows short fascicles of spindle cells that may display a vaguely storiform pattern, sometimes referred to as the “patternless” pattern

Fig. 5.47
On higher magnification, the cell population in an SFT is quite monotonous and composed of small spindle cells with dispersed chromatin, an absence of nucleoli, and an indistinct rim of amphophilic cytoplasm. Mitotic activity is very low to nil. Most authors will accept up to 3 mitoses per 10 high-power fields as the upper range acceptable for a diagnosis of benign SFT

Fig. 5.48
Immunohistochemical stains can be helpful to support the diagnosis of SFT. Most of the tumors will stain strongly positive for CD34 (40–70 %) and bcl-2 (80–100 %) (pictured) and will be negative for other differentiation markers such as cytokeratins, S-100 protein, actin, desmin, and epithelial membrane antigen (EMA)

Fig. 5.49
The recently developed STAT6 antibody has been claimed to be highly sensitive and specific for the diagnosis of SFT and shows nice nuclear positivity in most of the tumor cells. A small proportion of low-grade, dedifferentiated liposarcomas can also react with this antibody, requiring caution for its interpretation

Fig. 5.50
A constant theme observed in SFTs is stromal alterations, particularly stromal sclerosis and hyalinization, which most often result in a very striking picture characterized by parallel, ropelike strands of hyalinized collagen that are flanked by small spindle cells

Fig. 5.51
Higher magnification from Fig. 5.50 shows parallel arrays of hyalinized collagen showing a concentric arrangement around small vessels and flanked by flattened, small spindle cells

Fig. 5.52
Another unusual pattern of stromal collagenization in SFT is characterized by focal deposits of dense collagen fibers in a haphazard arrangement, although a perivascular arrangement around the lumen of small vessels is often apparent

Fig. 5.53
At higher magnification, the SFT in Fig. 5.52 shows a large cluster of irregularly deposited collagen fibrils adopting a somewhat stellate configuration. Structures such as these have been designated in the literature as “amianthoid” fibers and are composed of abnormal collagen

Fig. 5.54
Another form of stromal alteration in SFT is characterized by large, dilated vessels surrounded by a variously collagenized stroma, resulting in an angiofibromatous appearance. Notice the inconspicuous small spindle cells scattered within the surrounding stroma

Fig. 5.55
Stromal changes in SFT may also include prominent deposition of myxoid matrix simulating stromal edema. Such tumors can be easily mistaken for a variety of myxoid soft tissue tumors; identification of more solid and conventional areas of SFT will be helpful for making a correct diagnosis

Fig. 5.56
SFTs often can be extremely cellular. Such tumors are characterized by a tightly packed proliferation of spindle cells with very little stroma, closely resembling monophasic synovial sarcoma or dedifferentiated liposarcoma

Fig. 5.57
Higher magnification of solid SFT showing compact sheets of monotonous spindle cells circumscribing compressed vascular spaces. These tumors can be virtually indistinguishable morphologically from monophasic synovial sarcoma. Positivity for CD34 and STAT6 would favor SFT, whereas synovial sarcomas should show focal positivity for cytokeratins and EMA

Fig. 5.58
Another example of solid growth in SFT is characterized by a striking herringbone pattern with fascicles of spindle cells that appear to be branching from a central spine. Monophasic synovial sarcoma and MPNST can frequently display a similar growth pattern

Fig. 5.59
Areas of hypercellularity in SFT frequently can alternate with areas of hypocellularity. In fact, this variegation in growth patterns within the same tumor is quite distinctive for SFT and is a clue in distinguishing them from other spindle-cell soft tissue neoplasms

Fig. 5.60
Another area in an SFT showing partial stromal collagenization adjacent to areas with stromal edema and loose fibrous tissue

Fig. 5.61
Focal perivascular stromal collagenization is seen in this SFT. The spindle cells are very subtle and scant; the stroma shows extensive collagenization

Fig. 5.62
Another pattern of perivascular collagenization shows dilated vessels with open lumens surrounded by a population of spindle cells with ropelike, linear arrays of collagen

Fig. 5.63
Striking perivascular hyalinization is seen in this example of SFT of the mediastinum. The vessels are encased in a dense, cellular spindle-cell proliferation. The picture is reminiscent of a schwannoma

Fig. 5.64
At higher magnification, the SFT in Fig. 5.63 shows thick, concentric layers of hyalinized collagen surrounding the walls of small vessels

Fig. 5.65




Another distinctive feature of SFT is a vascular pattern characterized by a hemangiopericytic proliferation of small vessels with branching and open lumens, simulating hemangiopericytoma. It is now acknowledged that a significant number of cases previously diagnosed as hemangiopericytoma have corresponded to SFT with a striking hemangiopericytic pattern of growth
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


