Soft Tissue Pathology
Ligaments
Ligaments are connective tissue structures that connect bones. Their functions include guiding joint motion and stabilizing joints. Ligaments consist essentially of collagen, but the presence of neural structures of all types implies important proprioceptive function. Grossly, ligaments are white, dense, fibrillary structures with an avascular appearance, but they have an abundant vascular supply. They are relatively hypocellular and have abundant collagen within the tissue proper, which, like the tissue of tendons, also contains small proportions of elastin and glycosaminoglycans.
At the insertion of ligaments and tendons into bone, one may detect a more chondroid matrix, particularly after injury, such as rupture of the rotator cuff (Fig. 18.1). Both type I and type III collagen are present. Grossly, ligaments are variable in their appearance; they may be flat or cordlike. Fibers are often organized in a spiraling architectural pattern, similar to that of tendons. As interarticular structures, ligaments may be bathed in synovial fluid; a factor of importance in that ligaments may be subject to pathologic changes within the synovium in disorders such as rheumatoid arthritis and crystal deposition disease.
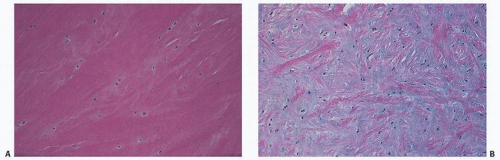
Microscopically, ligaments appear eosinophilic and are sparsely cellular, with the nuclei of fibroblasts seen as elongated basophilic structures lying between collagen bundles that are often lined up in columns (Fig. 18.2). Because of its birefringent property, collagen often appears as alternating bands, and its undulating characteristics, often referred to as crimp, give the impression of a crumpled or ruffled appearance. The nomenclature of ligaments is of interest in that names may be based on gross structural or functional features, as stated by Frank et al. (1). Therefore, the name of the acromial ligament refers to its bony attachment, the deltoid to its shape, the capsular ligaments to their gross function, the collateral ligaments to their relationship to a specific joint, and the cruciate ligaments to their relationship to each other.
Although the mechanical function of ligaments is generally accepted, their neurosensory or proprioceptive role is also significant, particularly in regard to joint replacement operations, during which these structures may be sacrificed. Research on the neurosensory role has uncovered within ligaments a broad range of neural receptors, which include the Ruffini receptors, mechanoreceptors found usually in the superficial layers of the joint capsule and on the surface of extrinsic ligaments; pacinian corpuscles, mechanoreceptors that appear as onionskin swirls in the deep layers of the joint capsule, particularly in the fibroadipose subsynovial tissue; Golgi receptors, found near the bone origins and insertions of ligaments and resembling Golgi tendon organs; and a fourth type—free, unmyelinated nerve endings thought to be related to pain (2).
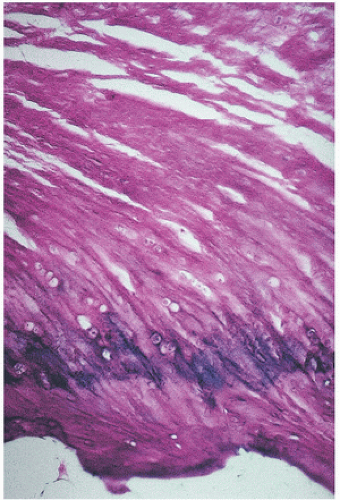 FIGURE 18.1. Ligament insertion into bone. Ligament (top) is eosinophilic and sparsely cellular except at insertion into bone, where chondroid cells appear. Note purple zone of calcification. |
Ligaments are essentially composed of collagen, which constitutes possibly three-fourths of their dry weight. The collagen is similar to that found in tendons, predominantly type I and less than 10 percent type III. Proteoglycans constitute less than 1 percent of the
dry weight, with other components of the ligament being elastin and noncollagenized glycoproteins such as fibronectin. Water constitutes approximately two-thirds of the wet weight of ligaments.
dry weight, with other components of the ligament being elastin and noncollagenized glycoproteins such as fibronectin. Water constitutes approximately two-thirds of the wet weight of ligaments.
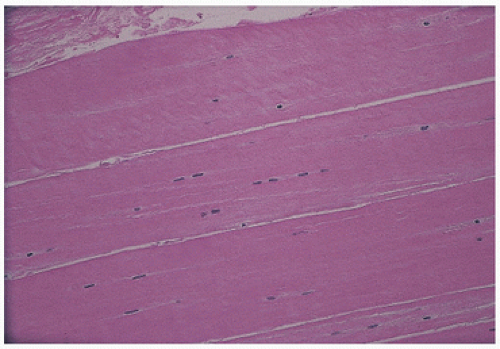 FIGURE 18.2. Ligament has eosinophilic cytoplasm and is sparsely cellular. Nuclei of fibroblasts often line up in columns. |
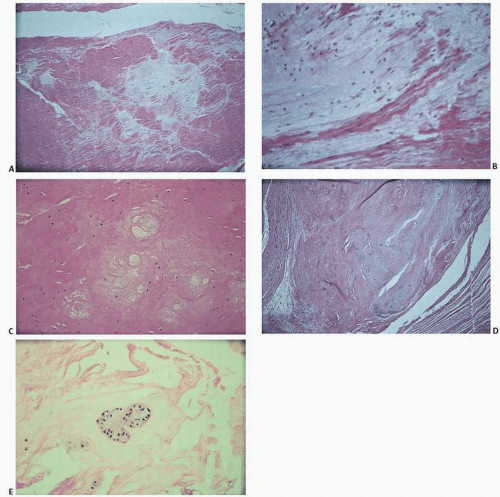
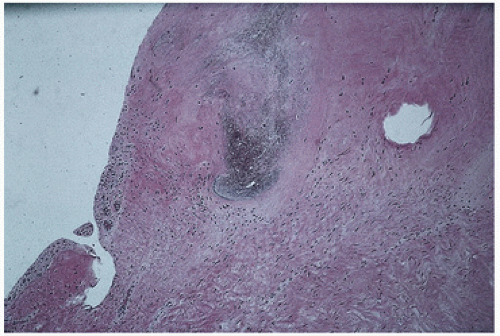 FIGURE 18.4. Ligament. Dark zone in center revealed calcium pyrophosphate crystals on polarized light. |
Injury or degeneration of ligaments can be seen microscopically as a broad range of changes (Fig. 18.3), including clearing or pallor of routine stains, looseness of the connective tissue matrix, cystic degeneration, chondroid metaplasia, and cartilage cloning.
Ligaments are not immune to the pathology that may afflict other joint structures. Osteoarthritic changes have been demonstrated in the ligament of the knee (3,4), and calcium pyrophosphate crystal deposition may be seen in a ligament (Fig. 18.4).
Although ligaments and tendons are biochemically similar, ligaments differ from tendons in being more metabolically active and possessing more plump cellular nuclei, a higher DNA content, larger amounts of reducible cross-links, more type III collagen, slightly less total collagen, and more glycosaminoglycans (5,6).
Tendons
Tendons connect muscle to bone for the purpose of transmitting forces to produce joint motion (7). They consist of spiraling bundles of predominantly Type I collagen fibers. Other collagen types in tendons include Types V and XII. However, tendons consist predominantly of an extracellular matrix that includes noncollagen components such as glycoproteins (e.g., cartilage oligomeric matrix protein, “COMP”) and proteoglycans (8).
The epitenon is a layer of fibroblasts or synovial-like cells that adhere to the surface. The tendon itself consists of longitudinally oriented type I collagen and mature fibroblasts. Anatomic features related to tendon function may give rise to abnormalities in clinical syndromes. For example, the cell surface consisting of synovial-like tissues may become hyperplastic and lead to the formation of a bursa-like space (see Fig. 15.3). The tendon sheath is the site of important pathology, such as giant cell tumor of tendon sheath and epithelioid sarcoma. Tendons possess one of the highest tensile strengths of body soft tissue, and their primary function is to transmit muscle force to the skeletal system, which depends on the microarchitecture of collagen and its associated elastin fibers. Elastin, usually less than 1 percent of the dry weight of tendons, is generally present in tendons at the fascicle surface. The morphologic characteristics and physiologic activity of cells at the surface of the tendon differ from those within the fascicle itself, and in fact different cell populations have been described on the tendon surface.
The vascular supply of the tendon is complex. Vessels extend into the tendon from a longitudinal direction and also enter at areas of synovial attachment, bony insertions, and others.
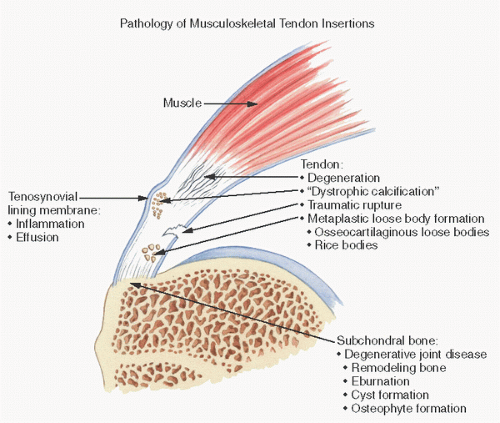
Tendon pathology is summarized in Figure 18.5.
In trauma-related tendon injuries, pathologic findings are subtle and may consist of abnormal fiber structure (waviness, nonparallel orientation), focal but usually sparse cellularity, vascular proliferation, rounded tenocytes, pyknotic nuclei, and occasionally fatlike inclusions.
Ligamentous and Tendinous Insertion into Bone
Both tendons and muscles constitute fibrillary connective tissue structures that insert into bone. The knee joint represents an example in which additional fibers may be seen inserting into the bone, most coursing through the periarticular or joint capsule tissue, the so-called joint capsule fibers. The attachment of these connective tissue structures into bone may be parallel to the orientation of the bone, or it may be somewhat branching or interwoven, the latter being the case with the cruciate ligaments.
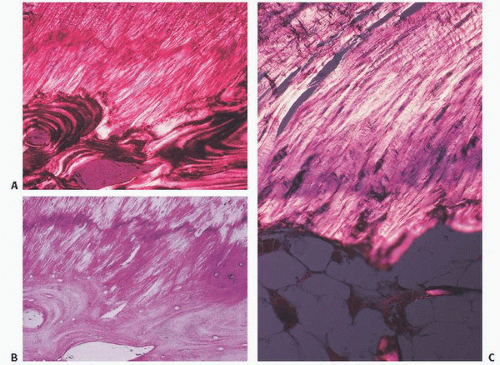
Microscopically, the insertion of both tendons and ligaments into bone can be divided into four distinct zones (Fig. 18.6). Zone I is the tendon or ligament itself. As described, these structures consist of collagen and proteoglycans with small amounts of elastin. They are well vascularized but probably poorly innervated, although numerous neural receptors have been identified in certain ligaments, such as the cruciate ligaments of the knee. As the tendon or ligament approaches the bone, there is distinct fibrocartilaginous change. Cells become larger with more rounded or oval chondrocyte-like nuclei. Eventually, chondrocytes are noted, with the tissue resembling that of fibrocartilage elsewhere, such as that of the meniscus. After trauma or injury, these insertions become more obviously chondroid in appearance, often showing chondrocyte cloning, such as that seen with damage to the meniscus of the knee.
The fibrocartilaginous tissue of zone II is where the tissue becomes mineralized. In zone III, a discernible line along the outer limits of calcification, the so-called tidemark or mineralization front, demarcates the zone of mineralization between articular cartilage and bone. This tidemark has a bluish or darkened appearance on routine hematoxylin and eosin staining, and, like that seen in the degenerating articular cartilage of degenerative joint disease, may widen or duplicate in areas of pathology, such as bunions in the foot. The biochemical changes that occur in zone III, the zone of calcified fibrocartilage, are presumably similar to those seen in the mineralized zone of articular cartilage.
Zone IV is bone. By using polarized light, one can see that the collagen fibrils of the tendon or ligament course directly into the bone.
Enthesopathies (Enthesopathic Syndromes)
Enthesopathies are characterized by clinical symptoms originating from sites of musculotendinous or ligamentous insertion into bone or soft tissue, such as fascial structures adjacent to bone (Table 18.1). Enthesopathy is a convenient term for an often bewildering array of
clinical syndromes with an etiology that is, for the most part, unknown. These conditions vary in degree of pain, extent of recognizable roentgenographic changes, and pathologic tissue changes.
clinical syndromes with an etiology that is, for the most part, unknown. These conditions vary in degree of pain, extent of recognizable roentgenographic changes, and pathologic tissue changes.
TABLE 18.1 Enthesopathies (Enthesopathic Syndromes) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Although they are commonly encountered in clinical practice, the pathology of these syndromes is usually nonspecific and rarely detailed in the literature.
Menisci
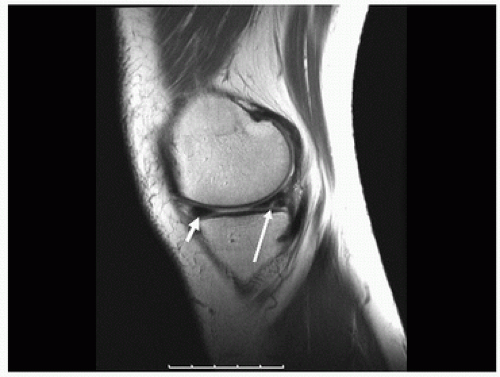
Menisci are curvilinear fibrocartilaginous structures that rest on the proximal articular surface of tibia in the knee joint (9) (Fig. 18.7). Although similar fibrocartilaginous joint structures occur in other locations, the menisci of the knee are most important because of the frequency with which they are injured during trauma. In the knee joint, the menisci create a concavity relative to the tibial plateau because their superior surface is concave and their inferior surface is flat. Each meniscus has a fine, tapered, semitranslucent inner edge and a broad-based peripheral rim where it attaches to the joint capsule, and each has a peripheral synovial coating that extends a short distance over the tibial and femoral surfaces and then becomes continuous with the joint capsule.
The medial meniscus is semilunar in shape, and the anterior and posterior horns are separated by approximately the length of the meniscus. The anterior horn is small, and the posterior horn is large with a broad base. The anterior horn of the medial meniscus is in close proximity to the infrapatellar fat pad. The entire meniscus is attached to the joint capsule.
 FIGURE 18.7. The menisci of the knee. The medial meniscus (right) is more semilunar in shape, whereas the lateral meniscus (left) is more discoid. |
The lateral meniscus is circular in shape, and the anterior and posterior horns are in close proximity. The anterior horn, midportion, and posterior horn are uniform in size. The anterior horn is adjacent to the infrapatellar fat pad. The meniscus is attached to the capsule except for the area posterior to the midportion, where the popliteal tendon crosses the joint through the popliteal hiatus.
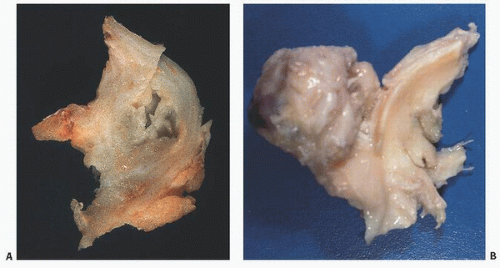
The menisci undergo marked color changes with age. In adolescents, the inner edge of the meniscus is translucent and beige (Fig. 18.8A), and in elderly persons, it is yellow-tan (Fig. 18.8B). Relatively avascular, the menisci are supplied by a perimeniscal capsular plexus derived from adjacent capsular and synovial tissue (10). Branches of the lateral, medial, and middle geniculate arteries supply blood.
Histologically, a meniscus is an interlaced network of collagen bundles (Fig. 18.9). The majority of these bundles of fibrocartilage are circumferentially oriented in the longitudinal axis and are interlaced with less obvious fibers oriented obliquely to the longitudinal axis. The circumferential fibers provide tensile strength, and the interlaced fibers provide resistance to compressive forces and splitting.
Biochemically, human meniscal tissue consists of approximately 70 percent collagen, 8 to 13 percent noncollagenous protein, and 1 percent hexosamine. There are four types of collagen in the meniscus; type I collagen predominates, making up about 90 percent of the total collagen. The predominance of type I collagen distinguishes fibrocartilage from hyaline cartilage, in which type II collagen predominates. The meniscus contains proteoglycans, mostly copolymeric dermatan sulfate and chondroitin sulfate chains. Proteoglycan content varies with age. The meniscus is sparsely populated by cells that synthesize and maintain the extracellular matrix and have been broadly categorized as two basic types: a fibroblast-like cell with a fusiform appearance, and an ovoid or polygonal cell. Little is known about these cells.
In recent studies, the neural supply to the meniscus was described as consisting of several types of nerve endings, including free nerve endings or corpuscle nerve organs. The former are located in the peripheral and medial thirds of the meniscus, and the latter are found exclusively in the anterior and posterior horns of the meniscus. The free nerve endings may constitute a pain receptor system, and it has been postulated that excision of an injured meniscus relieves pain through a denervation phenomenon (11). In addition, corpuscular types I, II, and III mechanoreceptors confirm deep sensitivity to the structure. The complex neural anatomy would suggest a proprioceptive function for this tissue. The meniscus also performs a broad range of biomechanical and other functions, including load bearing, shock absorption, joint lubrication, and joint stabilization.
Torn Meniscus
The most common abnormality of the meniscus is the occurrence of “tears,” ragged or peripheral serrated splitting of meniscal collagen fibers secondary to trauma. More than half a million arthroscopies are performed annually in the United States for meniscal injuries. Tears usually occur in young, active people, especially those engaged in running or contact sports. The classic clinical history in a torn meniscus is of a planted foot and a twisting or body-turning motion. A tear within the meniscus pathologically is an interruption of the smoothly contoured, curvilinear fibrocartilage. Given the complexity of the architecture of the meniscus, a disruption can occur in many planes. Once the substance of the meniscus is
interrupted, a vertical (longitudinal) or horizontal (transverse) cleavage plane may dissect in many directions. An initially vertically oriented cleft, for example, can easily dissect horizontally.
interrupted, a vertical (longitudinal) or horizontal (transverse) cleavage plane may dissect in many directions. An initially vertically oriented cleft, for example, can easily dissect horizontally.
 FIGURE 18.8. The meniscus is beige or translucent in youth (A) and yellows with age (B). Deposition of calcium pyrophosphate crystals causes whitening. |
Major categories of meniscal tears described by radiologists, surgeons, and pathologists are vertical or longitudinal tears, including the “parrot’s beak” and vertical “bucket handle” tears, a peripheral tear in which the meniscus is separated from the joint capsule, and horizontal or transverse tears (12) (Fig. 18.10). Tears may also occur with advancing age, particularly in persons older than 40 years. These so-called degenerative meniscal tears are horizontal, cleavage, flap-like tears. Traumatic injuries in younger persons usually cause either longitudinal or transverse vertical tears. The most frequent type of tear is a vertical longitudinal tear, with medial meniscal tears being more frequent. Peripheral tears more commonly cause symptoms. Less common are vertical transverse tears, which frequently involve the middle third of the lateral meniscus. In addition to tears, a common anatomic abnormality is a flattened, disc-like, or discoid meniscus (Fig. 18.11).
The menisci usually turn yellow with age because of lipid discoloration, and the discoloration corresponds to a decrease in pliability. Significant discoloration can indicate systemic metabolic or local metaplastic conditions. For example, the incidence of calcium pyrophosphate dihydrate crystals increases with age in the meniscus as well as the articular cartilage. These crystals are radiodense
and can be seen as punctate light calcifications on x-ray films and as fine, granular, white deposits on the surface and within the meniscus grossly (Fig. 15.22). White linear deposits may also occur in gout, but sodium urate is radiolucent on the x-ray film. Dystrophic calcification in the form of deposited hydroxyapatite can also occur, usually in the vessels in the outer third of the meniscus following trauma. Ossification can take place in meniscal structures (Fig. 18.12). A tan-brown or even a blackish green meniscus is commonly observed in patients with chronic hemarthrosis, as is seen in hemophilia. A blackish pigmented color is usually associated with ochronosis (Fig. 16.51).
and can be seen as punctate light calcifications on x-ray films and as fine, granular, white deposits on the surface and within the meniscus grossly (Fig. 15.22). White linear deposits may also occur in gout, but sodium urate is radiolucent on the x-ray film. Dystrophic calcification in the form of deposited hydroxyapatite can also occur, usually in the vessels in the outer third of the meniscus following trauma. Ossification can take place in meniscal structures (Fig. 18.12). A tan-brown or even a blackish green meniscus is commonly observed in patients with chronic hemarthrosis, as is seen in hemophilia. A blackish pigmented color is usually associated with ochronosis (Fig. 16.51).
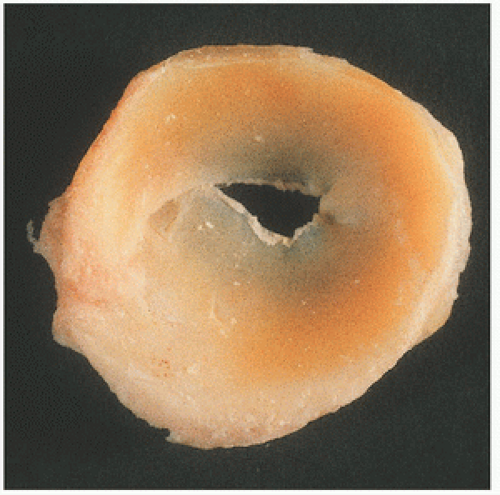 FIGURE 18.11. Most frequently, the lateral meniscus is discoid. Here, the discoid meniscus covers almost the entire tibial plateau. |
Histologically, a tear produces dissolution, loosening, and/or edematous change of the connective tissue matrix (Fig. 18.13). Occasionally, microcystic alterations or cartilaginous and osseous metaplasia may occur. These focal cystic or osseous chondral changes may be the predecessor of rarely observed cystic menisci or meniscal ossicles. The lateral meniscus more frequently becomes cystic, and the body or peripheral aspects of the meniscus may be affected (Fig. 18.14). With age, yellow discoloration increases, and fraying, particularly of the free meniscal edge, becomes more common. Proteoglycans increase with age, specifically the ratio of chondroitin-6-sulfate to chondroitin-4-sulfate. As mentioned, calcium pyrophosphate deposition also increases with aging.
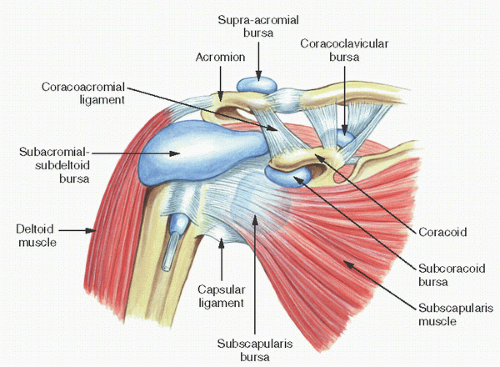
Bursae
A bursa is a space or potential space between anatomic planes (13) (Fig. 18.15). Unlike synovium, which it resembles, a bursa is normally lined by a sparsely cellular membrane and is not located within the diarthrodial space. It may be contiguous with the joint in pathologic states, as when involved by rheumatoid arthritis. Following a rotator cuff tendon tear, for example, the subacromial bursa may connect with the glenohumeral joint (14).
Bursae have a remarkable proliferative potential and are subject to the same alterations seen in synovium.
Bursal pathology, like synovial pathology, includes arthritis (15,16), infection, crystal deposition (gout and calcium pyrophosphate dihydrate deposition disease), hyperplasia, fluid secretion and cyst formation, chondromatosis (17), and pigmented villonodular synovitis (18).
Purported functions of bursae include reduction of friction where tendons move over bony prominences.
Bursae (or synovium) can form in pathologic states, as on the surface of an osteochondroma (19,20), in hallux valgus deformity, or at the interface between orthopaedic implants (screws, plates, total joint components).
One of the most extensively described bursae is that originating from the gastrocnemius-semimembranosus anatomic site, the reputed origin of the popliteal Baker cyst (21). Additionally, the historical orthopaedic literature is replete with folksy references to bursal pathology, including “housemaid’s knee,” “miner’s elbow,” and “weaver’s bottom” (14).
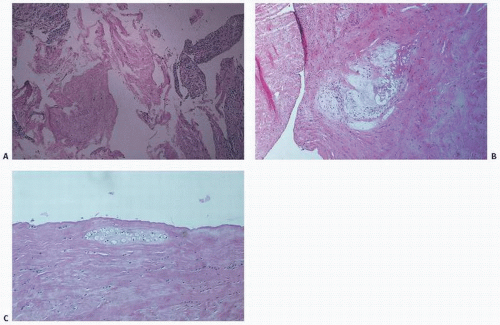 FIGURE 18.13. Histology of torn meniscus. (A) Fibrillation. (B) Loose connective tissue with microcystic degeneration. (C) Chondroid metaplasia. |
Microscopically, the bursa is a bland membrane lined by an intimal layer that is sparsely cellular (Fig. 18.16). Beneath the lining, as with synovium, is a variable amount of collagen and fat.
Although bursae normally contain little detectable fluid, the “lubricant” function and potential for fluid accumulation, as described and measured in pathologic bursal states, are self-evident.
There are more than 150 bursae in the body. A bursa is a sac lined with a synovium-like membrane that is normally present around joints or other areas subject to friction. Bursae serve to decrease friction and protect underlying structures from excessive pressure. Bursitis occurs when the fluid within the bursal sac becomes inflamed. The etiology is multifactorial. Trauma, immune
incompetence, infection, and iatrogenic and underlying systemic diseases may all cause bursitis.
incompetence, infection, and iatrogenic and underlying systemic diseases may all cause bursitis.
The most common sites of bursitis in the body are the most superficial areas. The subacromial, prepatellar, tibial collateral ligament, trochanteric, iliopsoas, olecranal, retrocalcaneal, and pes anserinus bursae are frequently affected clinically. Common complaints include tenderness with touch, swelling, pain on motion of the adjacent joint, warmth, and erythema. Bursitis occurs commonly with systemic diseases. Underlying conditions associated with bursitis include rheumatoid arthritis, scleroderma, gout, systemic lupus erythematosus, and immune incompetence (e.g., human immunodeficiency virus [HIV] infection, diabetes).
The majority of diagnoses of “bursitis” are made with a directed history and physical examination. Modalities such as magnetic resonance imaging (MRI) are helpful if the diagnosis is obscure.
The treatment of bursitis ranges from conservative, nonsurgical modalities to surgical excision. Treatment is focused toward the etiology. In septic bursitis (most common in the olecranal and prepatellar bursae), treatment includes aspiration with intravenous antibiotics and possibly bursectomy if symptoms persist. The most common bacterium isolated is Staphylococcus aureus.
It is critical to differentiate between septic and nonseptic bursitis. In infection, bursal swelling, pain, erythema, and tenderness are the usual signs and symptoms. Patients with septic bursitis seek medical attention sooner, are likely to be febrile, and are more likely to have a cellulitis of the surrounding skin. These findings, however, are not diagnostic of septic bursitis, so that an aspiration should be performed on most patients who present with superficial bursitis suspected of infection.
The treatment of nonseptic bursitis is symptomatic and includes administration of nonsteroidal anti-inflammatory drugs (NSAIDs). Aspiration and bursectomy are viable options for patients with more chronic conditions and with underlying systemic disorders. Steroid injections into the bursal sac have been shown to be beneficial in comparison with NSAIDs. Intrabursal tetracycline has been used, especially in cases of chronic olecranal bursitis associated with rheumatoid arthritis that have been resistant to steroid injection.
Bursectomy is considered the treatment of last resort for bursitis (septic and nonseptic). The initial treatment of bursitis is conservative. This includes aspiration, antibiotics (in cases of septic bursitis), steroid injection, and use of NSAIDs. Indications for bursectomy include septic bursitis refractory to aspiration and antibiotics (most common in the olecranal and prepatellar bursae), chronic bursitis refractory to the previously mentioned conservative modalities (subacromial, prepatellar, trochanteric, olecranal), and impending compression of surrounding vessels.
In general, surgical resection of the bursal sac is considered final; recurrence is very rare, with good treatment outcome.
Bursal chondromatosis is an idiopathic process characterized by bursal cell metaplasia into cartilage-forming cells. The pieces of cartilage may become detached from the bursa and form loose bodies (see Fig. 15.37D). The condition is distinct from osteochondritis dissecans and osteoarthritis, in which loose bodies are formed secondarily from articular cartilage pathology, including marginal osteophytes. By far the largest number of cases has been found to involve the popliteal bursa of the knee. Other sites include iliopectineal, bicipital, ankle, hip, subdeltoid, axillary, and elbow bursae.
Bursal chondromatosis is a very rare disorder. The exact etiology remains unknown, but possible factors include trauma, reactivation of residual embryonic cells, or possibly a benign neoplasm.
Pain, swelling, and limited range of motion are the most common clinical presentations. Radiographically, one sees multiple
radiopaque densities ranging in size from a few millimeters to several centimeters. Grossly, osteochondromatosis has the appearance of a glistening gray mass with multiple small, loose cartilaginous pieces. Histologic examination reveals lobules of hyaline cartilage with intervening fibrovascular septa lined by a thin layer of bursal cells at the periphery of the mass.
radiopaque densities ranging in size from a few millimeters to several centimeters. Grossly, osteochondromatosis has the appearance of a glistening gray mass with multiple small, loose cartilaginous pieces. Histologic examination reveals lobules of hyaline cartilage with intervening fibrovascular septa lined by a thin layer of bursal cells at the periphery of the mass.
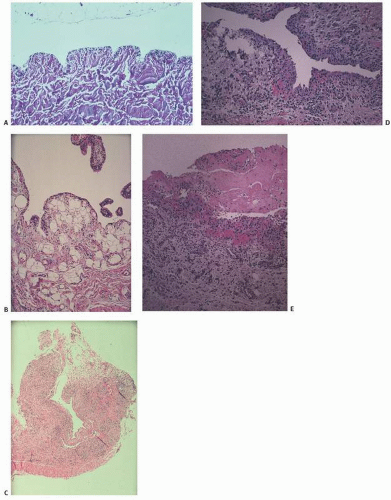 FIGURE 18.16. Bursal microscopy. (A) Membranous structure with sparse and inconspicuous lining. (B) Normal synovium for comparison. (C) Hyperplastic bursa with inconspicuous lining. (D) Hyperplastic bursal lining cells. (E) Fibrinous change.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|