CHAPTER 6 Smooth muscle and the cardiovascular and lymphatic systems
The cardiovascular system carries blood from the heart to all parts of the body through a series of tubes, all but the smallest of which are muscular. The muscle in these tubes is of two types: smooth muscle is characteristic of the walls of blood vessels and cardiac muscle provides the walls of the heart chambers with their powerful contractile pumping action. The general characteristics and classification of muscle tissues are given on page 103. Smooth muscle also forms an important contractile element in the walls of many other organ systems of the body, e.g. the gastrointestinal tract.
SMOOTH MUSCLE
Smooth muscle cells (fibres) are smaller than those of striated muscle. Their length can range from 15 μm in small blood vessels to 200 μm, and even to 500 μm or more in the uterus during pregnancy. The cells are spindle-shaped, tapering towards the ends from a central diameter of 3–8 μm (Fig. 6.1). The nucleus is single, located at the midpoint, and often twisted into a corkscrew shape by the contraction of the cell. Smooth muscle cells aggregate with their long axes parallel and staggered longitudinally, so that the wide central portion of one cell lies next to the tapered end of another. Such an arrangement achieves both close packing and a more efficient transfer of force from cell to cell. In transverse section, smooth muscle is seen as an array of circular or slightly polygonal profiles of very varied size, and nuclei are present only in the centres of the largest profiles (Fig. 6.2). This appearance contrasts markedly with that of skeletal muscle cells, which show a consistent diameter in cross-section and peripherally placed nuclei throughout their length.
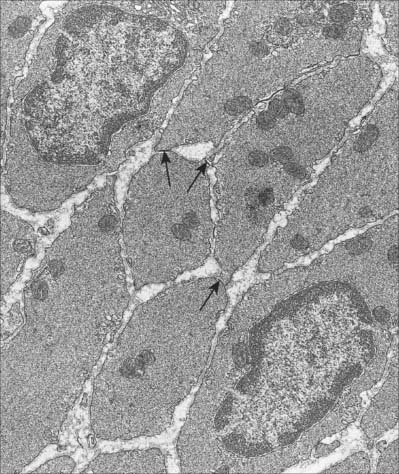
Smooth muscle has no attachment structures equivalent to the fasciae, tendons and aponeuroses associated with skeletal muscle. There is a special arrangement for transmitting force from cell to cell and, where necessary, to other soft tissue structures. Cells are separated by a gap of 40–80 nm. Each cell is covered almost entirely by a prominent basal lamina which merges with a reticular layer consisting of a network of fine elastin, reticular fibres (collagen type III) and type I collagen fibres (Fig. 6.3). These elements bridge the gaps between adjacent cells and provide mechanical continuity throughout the fascicle. The cell attaches to components of this extracellular matrix at dense plaques (see Fig. 6.4A) where the basal lamina is thickened; cell–cell attachment occurs at intermediate junctions or desmosomes, formed of two adjacent dense plaques. At the boundaries of fascicles, the connective tissue fibres become interwoven with those of interfascicular septa, so that the contraction of different fascicles is communicated throughout the tissue and to neighbouring structures. The components of the reticular network, the ground substance and collagen and elastic fibres, are synthesized by the smooth muscle cells themselves, not by fibroblasts or other connective tissue cells, which are rarely found within fasciculi.
Discontinuities occur in the basal lamina between adjacent cells, and here the cell membranes approach to 2–4 nm of one another to form a gap junction (Fig. 6.2). These junctions are believed to be structurally similar to their counterparts in cardiac muscle. They provide a low-resistance pathway through which electrical excitation can pass between cells, producing a coordinated wave of contraction. The incidence of gap junctions varies with the anatomical site of the tissue: they appear to be more abundant in the type of smooth muscle which generates rhythmic (phasic) activity.
MICROSTRUCTURE OF SMOOTH MUSCLE AND THE CONTRACTILE MECHANISM
Although electron microscopy revealed the presence of filaments in smooth muscle some years ago, this observation alone provided little insight into their mode of function because of the lack of any obvious organization of the filaments. More recent work, using high-resolution immunocytochemistry, has revealed further details of the internal architecture of the cell and suggests a structural basis for contractile function. The model, which is illustrated in Fig. 6.3, depends on the mutual interaction of two systems of filaments, one forming the cytoskeleton and the other the contractile apparatus.
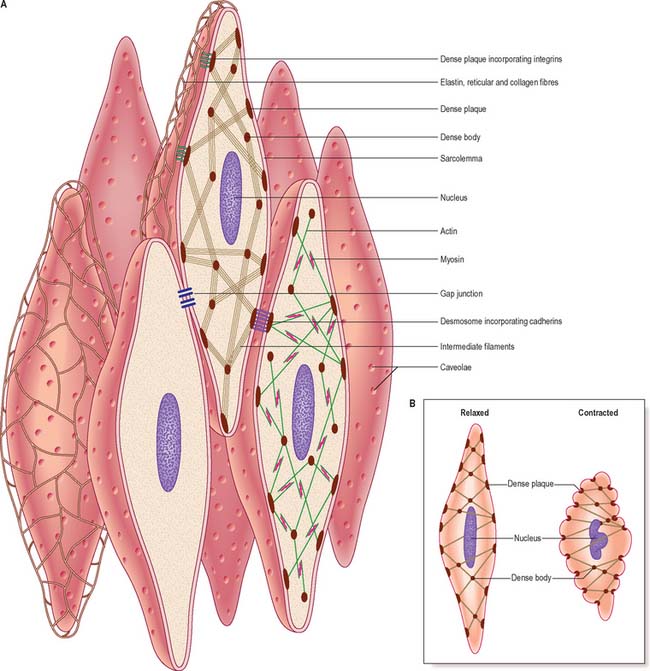
Excluding the perinuclear region, the cytoplasm of a smooth muscle cell effectively consists of two structural domains. The cytoskeleton forms a structural framework that maintains the spindle-like form of the cell and provides an internal scaffold with which other elements can interact. Its major structural component is the intermediate filament desmin, with the addition of vimentin (which may also be present alone) in vascular smooth muscle. The intermediate filaments are arranged mainly in longitudinal bundles, but some filaments interconnect the bundles with each other and with the sarcolemma to form a three-dimensional network. The bundles of intermediate filaments insert into focal, electron-dense bodies, approximately 0.1 μm in diameter, which are distributed uniformly throughout the cytoplasm and also attach to dense plaques underlying the plasma membrane (Fig. 6.3A). The cytoplasmic dense bodies and submembraneous dense plaques are equivalent to the Z-discs of striated muscle cells. They contain the actin-binding protein α-actinin and thus also anchor the actin filaments of the contractile apparatus. These form a lattice of obliquely arranged bundles throughout the cytoplasm, which transmit force to the plasma membrane and thus the basal lamina and extracellular matrix via dense plaques. These are associated with a highly structured arrangement of ancillary proteins, including vinculin and talin, which in turn attach to integrins that cross the membrane and provide attachment to components of the extracellular matrix. An analogous arrangement underlies cell–cell attachment at desmosomes, but here the attachment between dense plaques is provided by transmembrane cadherin glycoproteins and intracellular catenins instead of integrins and talin. Mechanical deformation of the cell may be linked to cell signalling mechanisms via focal adhesion kinase (FAK) and its substrate paxillin; phosphorylation of talin and paxillin may modulate the deformability of the smooth muscle cell. Other regulatory proteins also associate specifically with actin, such as caldesmon and calponin. The cytoskeleton is therefore not a passive structure, and can adapt dynamically to load, presumably therefore contributing to the low energy requirements of smooth muscle contraction. The ratio of actin to myosin is about eight times greater in smooth compared to striated muscle, reflecting the greater length of actin filaments in smooth muscle.
Smooth muscle myosin filaments are 1.5–2 μm long, somewhat longer than those of striated muscle. Although smooth muscle cells contain less myosin, the longer filaments are capable of generating considerable force. The myosin filaments of smooth muscle are also assembled differently, such that their head regions lie symmetrically on either side of a ribbon-like filament, rather than imposing a bipolar organization on the filament. Actin filaments, to which they bind, can thus slide along the whole length of the myosin filament during contraction. This difference underpins the ability of smooth muscle to undergo much greater changes in length than striated muscle. Actin–myosin filament sliding generates tension which transmits to focal regions of the plasma membrane, changing the cell to a shorter, more rounded shape (Fig. 6.3B) and often deforming the nucleus to a corkscrew-like profile.
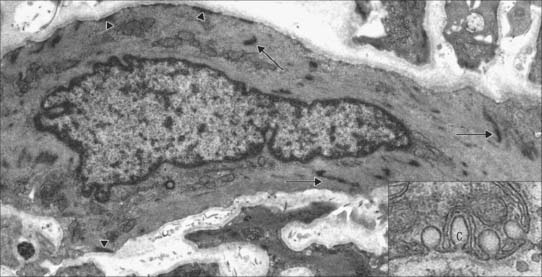
Caveolae, cup-like invaginations of the plasma membrane with a resemblance to endocytotic vesicles, are a characteristic feature of smooth muscle cells, and may form up to 30% of the membrane (Fig. 6.4). They are associated with many receptors, ion channels and kinases, and the peripheral sarcoplasmic reticulum and may thus act as sites for highly localized signalling pathways. They may also act as specialized pinocytotic structures involved in fluid and electrolyte transport into the cell. Other organelles (mitochondria, ribosomes etc) are largely confined to the filament-free perinuclear cytoplasm, although in some smooth muscle types, including vascular smooth muscle, peripheral mitochondria, sarcoplasmic reticulum and sarcolemma seem to form signalling microdomains. Recent studies using mitochondria-specific staining of such smooth muscle cells often show mitochondria forming a reticular network within the cytosol, which would be consistent with a cell signalling function, especially that concerned with intracellular calcium homeostasis.
INNERVATION
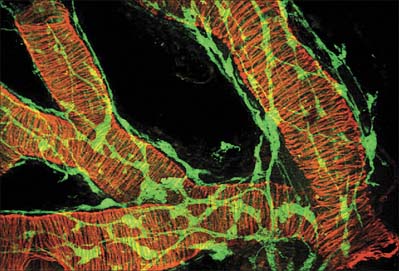
Smooth muscles are innervated by unmyelinated axons whose cell bodies are located in autonomic ganglia, either in the sympathetic chain or, in the case of parasympathetic fibres, closer to the point of innervation (Fig. 6.5). They ramify extensively, spreading over a large area of the muscle and sending branches into the muscle fasciculi. The terminal portion of each axonal branch is beaded, and consists of expanded portions, varicosities, packed with vesicles and mitochondria, separated by thin, intervaricose portions. Each varicosity is regarded as a transmitter release site, and, in the functional sense, is therefore a nerve ending. In this way the axonal arborization of a single autonomic neurone bears a very large number of nerve endings (up to tens of thousands), as opposed to a maximum of a few hundred in somatic motor neurones. The neuromuscular terminals of autonomic efferents are considered in more detail on page 62.
The neuromuscular junctions in smooth muscles do not show the consistent appearance seen in skeletal muscles. The neurotransmitter diffuses across a gap that can vary from 10 to 100 nm: even separations up to 1 μm may still allow neuromuscular transmission to take place, although more slowly. The nerve ending is packed with vesicles, but the adjacent area of the muscle cell is not structurally differentiated from that of non-junctional regions – there is no distinct synapse.
EXCITATION–CONTRACTION COUPLING IN SMOOTH MUSCLE
The regulation of contraction of smooth muscle is however largely calcium-dependent. In the cytoplasm, calcium binds to calmodulin. The complex so formed regulates the activity of myosin light chain kinase, which phosphorylates myosin regulatory light chains and initiates the myosin-actin ATPase cycle (p. 111). The enzymatic activation process is therefore inherently slow. Unphosphorylated myosin II of smooth muscle cannot initiate actin binding, although it can maintain contraction, with little energy expenditure. Myosin phosphatase dephosphorylates myosin light chains, and thus promotes relaxation. Inhibition of the phosphatase, for example by Rho kinase, increases phosphorylation for any level of calcium (i.e. increases calcium sensitivity). This is now believed to be a very important component of the response to many constrictor agonists.
DEVELOPMENT
It was thought that all smooth muscle cells developed in situ exclusively from the splanchnopleuric mesenchyme in the walls of the anlagen of the viscera and around the endothelium of blood vessels. However, recent experimental studies have traced the progeny of cells proliferating from the epithelial plate of the somite and have identified endothelial and tunica media smooth muscle cells arising from individual somites (Scaal & Christ 2004). The origin of the smooth muscle of the iris is still unclear. This region of the eye develops from the optic cup, and so the smooth muscle which arises there is derived either from the neurectoderm of the original optic cup or from the neural crest mesenchyme which later invades the iris.
THE CARDIOVASCULAR AND LYMPHATIC SYSTEMS
GENERAL ORGANIZATION
From the centre to the periphery, the vascular tree shows three main modifications. The arteries increase in number by repeated bifurcation and by sending out side branches, in both the systemic and the pulmonary circulation. For example, the aorta, which carries blood from the heart to the systemic circulation, gives rise to about 4 × 106 arterioles and four times as many capillaries. The arteries also decrease in diameter, although not to the same extent as their increase in number, so that a hypothetical cross-section of all the vessels at a given distance will increase in total area with increasing distance from the heart. At its emergence from the heart, the aorta of an adult man has an outer diameter of approximately 30 mm (cross-sectional area of nearly 7 cm2). The diameter decreases along the arterial tree until it is as little as 10 μm in arterioles (each with a cross-sectional area of about 80 μm2). However, given the enormous number of arterioles, the total crosssectional area at this level is approximately 150 cm2, more than 200 times that of the aorta. As a result, blood flow is faster near the heart than at the periphery.
The development of blood vessels is described on pages, 206–208.
General features of vessel walls
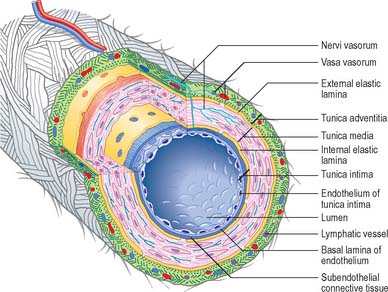
Blood vessels, irrespective of size, and with the exception of capillaries and venules, have walls consisting of three concentric layers (tunicae) (see Fig. 6.7). The intima (tunica intima), is the innermost layer. Its main component, the endothelium, lines the entire vascular tree, including the heart, and the lymphatic vessels. The media (tunica media) is made of muscle tissue, elastic fibres and collagen. While it is by far the thickest layer in arteries, the media is absent in capillaries and is comparatively thin in veins. The adventitia (tunica adventitia) is the outer coat of the vessel, and consists of connective tissue, nerves and vessel capillaries (vasa vasorum). It links the vessels to the surrounding tissues. Vessels differ in the relative thicknesses and detailed compositions of their layers and, in the smallest vessels, the number of layers represented.
Large elastic arteries
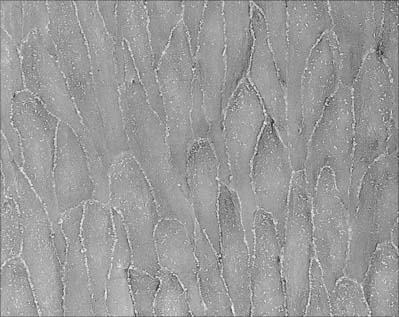
The intima is made of an endothelium, resting on a basal lamina, and a subendothelial connective tissue layer. The endothelial cells are flat, elongated and polygonal in outline, with their long axes parallel to the direction of blood flow (see Fig. 6.17). The subendothelial layer is well developed, contains elastic fibres and type I collagen fibrils, fibroblasts and small, smooth muscle-like myointimal cells. The latter accumulate lipid with age and in an extreme form, this feature contributes to atherosclerotic changes in the intima. Thickening of the intima progresses with age and is more marked in the distal than in the proximal segment of the aorta.
A prominent internal elastic lamina, sometimes split, lies between intima and media. This lamina is smooth, measures about 1 μm in thickness, and, with the elastic lamellae of the media, is stretched under the effect of systolic pressure, recoiling elastically in diastole. Elastic arteries thus have the effect of sustaining blood flow despite the pulsatile cardiac output. They also smooth out the cyclical pressure wave. The media has a markedly layered structure, in which fenestrated layers of elastin (elastic lamellae) alternate with interlamellar smooth muscle cells (Fig. 6.6), collagen and fine elastic fibres. The arrangement is very regular, such that each elastic lamella and adjacent interlamellar zone is regarded as a ‘lamellar unit’ of the media. In the human aorta there are approximately 52 lamellar units, measuring about 11 μm in thickness. Number and thickness of lamellar units increases during postnatal development, from 40 at birth.
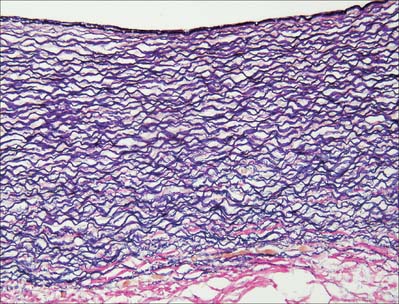
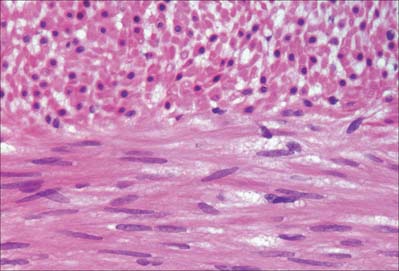
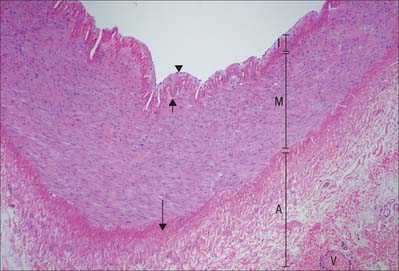
Fig. 6.6 Elastic artery (human aorta), stained for elastic fibres. The dense staining of the internal elastic lamina is seen close to the luminal surface (top); elastic lamellae fill the tunica media and merge with the external elastic lamina at its junction with the collagenous adventitia (red fibres, below). Compare with Fig. 6.20. Elastic van Gieson technique.
Muscular arteries
Muscular arteries are characterized by the predominance of smooth muscle in the media (Fig. 6.8). The intima consists of an endothelium, similar to that of elastic arteries, which rests on a basal lamina and subendothelial connective tissue. The internal elastic lamina (Fig. 6.7, Fig. 6.8) is a distinct, thin layer, sometimes duplicated and occasionally absent. It is thrown into wavy folds as a result of contraction of smooth muscle in the media. Some 75% of the mass of the media consists of smooth muscle cells which run spirally or circumferentially around the vessel wall. The relative amount of extracellular matrix is therefore less than in large arteries, however, fine elastic fibres which run mainly parallel to the muscle cells are present. An external elastic lamina, composed of sheets of elastic fibres, forms a less compact layer than the internal lamina, and separates the media from the adventitia in the larger muscular arteries. The adventitia is made of fibroelastic connective tissue, and can be as thick as the media in the smaller arteries. The inner part of the adventitia contains more elastic than collagen fibres.
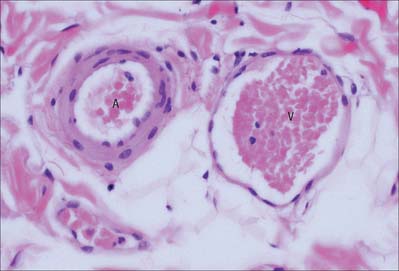
Arterioles
In arterioles (Fig. 6.9, Fig. 6.10) the endothelial cells are smaller than in large arteries, but their nuclear region is thicker and often projects markedly into the lumen. The nuclei are elongated and orientated parallel to the vessel length, as is the long axis of the cell. The basal surface of the endothelium contacts a basal lamina, but an internal elastic lamina is either absent or is highly fenestrated and traversed by the cytoplasmic processes of muscle cells or endothelial cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree