ANATOMY
The small intestine is the portion of the alimentary tract extending from the pylorus to the ileocecal valve and it consists of three segments—the duodenum, the jejunum (upper two-fifths), and the ileum (lower three-fifths). The anatomy, physiology, and pathology of the duodenum are discussed in Chapter 23.
The jejunum begins at the ligament of Treitz. The jejunum and ileum are suspended on a mobile mesentery covered by a visceral peritoneal lining that extends onto the external surface of the bowel to form the serosa. There is no sharp demarcation between the jejunum and the ileum; as the intestine proceeds distally, the lumen narrows, the mesenteric vascular arcades become more complex, and the circular mucosal folds become shorter and fewer.
The mesentery contains fat, blood vessels, lymphatic channels and nodes, and nerves. The jejunum and ileum are supplied by the superior mesenteric artery (SMA). Branches within the mesentery anastomose to form arcades, and small straight arteries from these arcades enter the mesenteric border of the gut. Venous blood is drained through the superior mesenteric vein (SMV), which then joins the splenic vein behind the pancreas to form the portal vein.
Lymphatic drainage is abundant. Elliptical, lymphoid aggregates (Peyer’s patches) are present in the submucosa on the antimesenteric border along the distal ileum, and smaller follicles are evident throughout the remainder of the small intestine. Regional lymph nodes follow vascular arcades and drain toward the cisterna chyli.
The nerve supply for the small bowel is both sympathetic (fibers from the greater and lesser splanchnic nerves) and parasympathetic (from the right vagus nerve). Although both types of autonomic nerves contain efferent and afferent fibers, only the sympathetic afferents appear to mediate intestinal pain.
The wall of the small intestine consists of four layers—mucosa (innermost), submucosa, muscularis, and serosa (outermost).
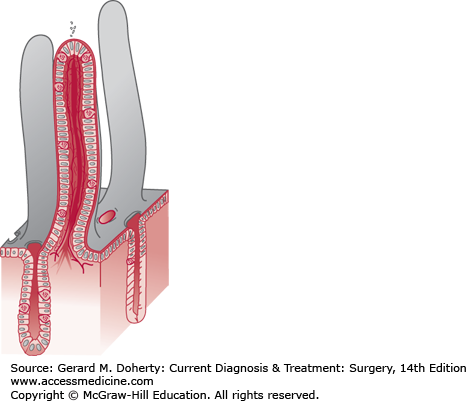
The mucosa is characterized by circular folds about 10 mm high, named valvulae conniventes, that are taller and more numerous in the proximal jejunum and project into the lumen (Figure 29–1). These folds, combined with the presence of villi on the surface of the valvulae conniventes, increase the absorptive surface area about eight times. There are approximately 20-40 villi/mm2. They are about 0.5-1 mm long and their walls are made up of epithelial cells with tiny projections named microvilli. The epithelial cells enclose a central axis that contains an arteriole surrounded by blood and lymphatic capillaries, known as a lacteal, and fibers from the muscularis mucosae. The microvilli (1 μm in height) amplify the potential absorptive surface area up to 200-500 m2 (Figure 29–2).
Figure 29–1.
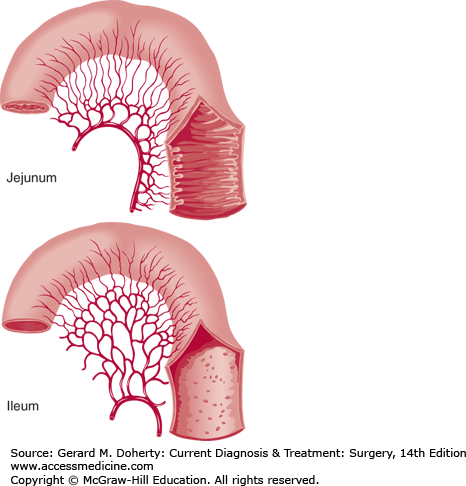
Blood supply and luminal surface of the small bowel. The arterial arcades of the small intestine increase in number from one or two in the proximal jejunum to four or five in the distal ileum, a finding that helps to distinguish proximal from distal bowel at operation. Plicae circulares are more prominent in the jejunum.
The mucosa is microscopically subdivided into three different layers: (1) the muscularis mucosae, the outermost, consists of a thin sheet of smooth muscle cells; (2) the lamina propria consists of connective tissue that extends from the base of the crypts up into the intestinal villi; and (3) the epithelium which is the innermost layer.
Intestinal epithelium in composed of multiple cell types that rest on a thin basement membrane overlying the lamina propria. There are two major compartments to the intestinal epithelium, the crypt and the villus, each with distinct function and cellular composition. The crypt is populated by cells that are predominantly secretory and which derive from a pluripotent stem cell located above the base of the crypts of Lieberkühn. Paneth cells stay at the base of the crypts; their function is still unknown but may be secretory, as resembling zymogen-secreting cells of the pancreas.
Most of the crypt cells are undifferentiated; some mature into mucus-secreting goblet cells and enteroendocrine cells, but the majority become absorptive enterocytes. Enteroendocrine cells include enterochromaffin cells (the most common), N cells that contain neurotensin, L cells (glucagon), and motilin and cholecystokinin (CCK) containing cells. Finally, M cells and T lymphocites play a major role in mucosal cell-mediated immunity.
The villus compartment is nonproliferative. Factors that affect enterocyte differentiation include growth factors, hormones, matrix proteins, and luminal nutrients. The life span of enterocytes is 3-6 days.
The submucosa is a dense connective tissue layer populated by different cell types, including fibroblasts, mast cells, lymphocytes, macrophages, eosinophils, and plasma cells. It contains blood vessels, lymphatics, and nerves. Meissner’s submucosal neural plexus interconnects with neural elements from Auerbach’s plexus. The submucosa is the strongest layer of small bowel wall. The muscularis consists of two layers of smooth muscle, a thicker inner circular layer and a thinner outer longitudinal layer. Specialized intercellular junctional structures called gap junctions electrically couple adjacent smooth muscle cells and allow efficient propagation of peristalsis. Ganglion cells and nerve fibers of Auerbach’s myenteric plexus interdigitate between layers and communicate with smaller neural elements between cells. The serosa consists of a single layer of flattened mesothelial cells that covers the small bowel.
PHYSIOLOGY
Intestinal motility consists of propulsion of luminal contents (peristalsis) combined with mixing action through segmentation. These functions are accomplished by both the outer longitudinal and inner circular muscle layers of the intestinal wall, mainly under the direct control of the myoenteric nervous plexus. The submucosal nervous plexus is primarily involved in the regulation of secretion and absorption. The extrinsic sympathetic input is excitatory and the peptidergic input likely inhibitory. Intestinal motility is also under positive control by local hormones such as motilin and CCK. Smooth muscles of the small intestine undergo spontaneous oscillations of membrane potentials, known as pacesetter potentials, with progressively decreasing frequency from the duodenum to the ileum. The frequency of pacesetter potentials for the entire small intestine is determined by the duodenum, where they originate. A nerve-related cell type known as the interstitial cell of Cajal appears to play a key role in the generation of pacemaker activity. Small bowel motility varies with the fasted and fed state. During the interdigestive or fasting period, a cyclical pattern of motor activity consisting of three phases is observed. Phase I is resting and lasts for about 80% of the cycle. Phase II, about 15%, consists of random contractions of moderate amplitude. Phase III, about 5%, is a series of brief high pressure waves. This three-phase cycle results in a pattern called the migrating motor complex, which is abolished by ingestion of food. In the fed state, the pattern of contraction is more frequent and consistent over time. Rather than beginning from a proximal site and propagating distally, contractions begin at all levels along the small bowel and spread distally.
The intestinal epithelium selectively limits the permeation of potentially harmful luminal substances. The anatomical barrier is the intercellular junction complex, a three-level structure that forms a circumferential seal between adjacent cells: the tight junction faces the lumen, the intermediate junction lies deep to the tight junction, and the desmosome is the innermost element of this complex. Several pathological conditions can alter the barrier function. Certain bacterial toxins, such as Clostridium difficile, directly perturb the barrier function through disruption of cytoskeletal-junctional interactions, and various cytokines and proinflammatory mediators can also modulate intestinal permeability.
Digestion begins in the stomach with the action of gastric acid and pepsin. In the proximal duodenum, ingested food is broken down by pancreatic enzymes such as trypsin, elastase, chymotrypsin, and carboxypeptidases. The activity of intestinal hydrolases and olygopeptidases then accomplishes terminal protein and carbohydrate digestion, and the resulting monosaccharides, amino acids, or di- and tripeptides then serve as substrates for Na+– or H+-coupled transporters in the apical membrane of absorptive enterocytes. Fat digestion and absorption occur in the proximal small bowel, where pancreatic lipase partially hydrolyzes triglycerides into two fatty acids and a monoglyceride. These substances are solubilized by bile salts to form micelles that diffuse into enterocytes releasing fatty acid and monoglyceride. Triglycerides are transported intracellularly and incorporated along with cellular protein, phospholipid, and cholesterol to form chylomicrons. They then exit the cell to be absorbed by the lymphatic system. Bile salts are reabsorbed into the enterohepatic circulation in the distal ileum by an ileal Na+-coupled bile acid transporter.
The small bowel receives about 1-1.5 L/day of ingested fluids and about 8 L of salivary, gastric, and pancreatico-biliary secretions. Most of this fluid is reabsorbed before reaching the colon. Water movement is driven by the active transcellular absorption of Na+ and Cl− and by absorption of nutrients such as glucose and amino acids. The energy for many of these processes derives from the activity of a Na+-K+ ATPase, that maintains the low Na+ internal environment that drives uptake via coupled ion exchangers (Na+/H+ and Cl−/HCO3−) and Na+-coupled nutrient transporters.
Intestinal crypt cells secrete an isotonic fluid through the active transcellular transport of Cl−. This process lubricates the mucosal surface and facilitates the luminal extrusion of other secrete substances. Diarrhea results when secretion exceeds intestinal absorptive capacity.
The mucosal immune system is extremely important in defense against toxic and pathogenic threats from the luminal environment. The lamina propria contains numerous immune cells including plasma cells, mast cells, and lymphocytes that produce both immunoglobulins and cytokine mediators.
Plasma cells produce IgA in response to food antigens and microbes. IgA and IgM are secreted into the lumen by a mechanism that involves transcytosis through epithelial cells after binding to the polymeric immunoglobulin receptor on the basolateral membrane. Secretory IgA prevents microbial pathogens from penetrating the epithelial layer. IgA-antigen interactions also occur within the intraepithelial and subepithelial compartments. Intestinal epithelial cells themselves may also contribute to the immune function of the bowel. These cells express major histocompatibility class I and class II molecules on their surface and may function as weak antigen-presenting cells. The epithelial cell layer may transmit important immunoregulatory signals to the underlying lymphocytic population.
Specialized cells known as M cells are found overlying Peyer’s patches and act as the major portal of entry for foreign bodies. Specialized membrane invaginations in these cells create a pocket in which lymphocytes and macrophages gather. Luminal substances are immediately delivered to these antigen-presenting cells, and this information is directly conveyed to the underlying follicles. Intraepithelial lymphocytes (IEL) are specialized T cells that reside in the paracellular space between absorptive enterocytes. The precise role of IELs is still uncertain, but they may mediate cross-talk between epithelial cells and the underlying immune and nonimmune cells of the lamina propria. Within the lamina propria and submucosa, mature T cells, B cells, and macrophages carry out traditional cell-mediated immune response including phagocytosis, cell killing, and cytokine secretion. Mucosal and connective tissue mast cells produce numerous mediators that contribute to overall immune response and modulate the many functions of the epithelial cells.
The small bowel is a rich source of regulatory peptides that control various aspects of gut function. These substances, released in response to luminal or neural stimuli, exert their biological actions either at distant sites or locally.
Secretin is a 27-amino-acid peptide released by enteroendocrine cells in the proximal small bowel in response to luminal acidification, bile salts, and fat. Its major function is to stimulate pancreatic ductal alkaline secretion. Secretin inhibits gastric acid secretion, and gastrointestinal motility. In addition, it stimulates bile flow by stimulating fluid secretion from cholangiocytes. Other members of the secretin family that share substantial sequence homology and interact with similar receptors include vasoactive intestinal polypeptide (VIP), glucagon, gastric inhibitory polypeptide (GIP), and enteroglucagon. Enteroglucagon and glucagon-like peptides are secreted by neuroendocrine cells in the colon and small bowel and may play an important role in gut adaptation and glucose homeostasis.
CCK is released by specialized enteroendocrine cells in response to luminal amino acids and medium- to long-chain fatty acids. CCK release is inhibited by intraluminal trypsin and bile salts. Two major targets of CCK are the gallbladder and the sphincter of Oddi, where it causes coordinated contraction and relaxation, respectively, to enhance luminal mixing of bile with ingested food. Furthermore, CCK stimulates pancreatic enzyme secretion and cell growth in intestinal mucosa and the pancreas, insulin release and intestinal motility.
Somatostatin is a 14-amino-acid peptide that exerts a wide variety of inhibitory functions in the gastrointestinal tract. It is released from specialized enteroendocrine cells and it acts in paracrine fashion to inhibit intestinal, gastric, and pancreatico-biliary secretion and cell growth. Synthetic forms of somatostatin are used in the clinical practice in patients with enterocutaneous and pancreatico-biliary fistulae.
Peptide YY is a 36-amino-acid peptide secreted by the distal small bowel and it inhibits gastric acid and pancreatic secretion, as well as several intestinal hormones, and decreases intestinal motility.
Motilin is secreted by the duodenum and the proximal jejunum, where it acts to enhance contractility and accelerate gastric emptying.
Neurotensin is produced in the ileum and enteric nerves; it appears to affect a variety of enteric functions including gastric acid secretion, gastric emptying, intestinal motility, and secretion.
Other peptides (VIP, calcitonin-related peptide, galanin, bombesin, neuropeptide Y, gastrin-releasing peptides, and substance P) are released from enteric nerves, but their precise role has not been fully clarified.
SMALL BOWEL OBSTRUCTION
Small bowel obstruction (SBO) is one of the most common disorders affecting the small bowel. It is characterized by impairment in the normal flow of intraluminal contents and can be divided into mechanical obstruction and paralytic ileus.
Mechanical obstruction implies an extrinsic or intrinsic obstacle that prevents the aboral progression of intestinal contents and it may be complete or partial. Simple obstruction occludes the lumen only; obstruction with strangulation impairs the blood supply also and leads to necrosis of the intestinal wall. Paralytic (or adynamic) ileus is due to a neurogenic failure of peristalsis to propel intestinal contents with no mechanical obstruction.
The causes of mechanical obstruction can be divided into three groups according to the relationship to the intestinal wall: (1) intraluminal; (2) intramural; and (3) extrinsic. The three most common etiologies are intra-abdominal adhesions, hernias, and neoplasms (Table 29–1).
Adhesions Sixty to 75% of cases of mechanical SBO are secondary to adhesions related to prior abdominal surgery. Lower abdominal and pelvic surgery appears to be associated with a higher incidence of adhesions compared to upper abdominal surgery. Congenital bands are rarely seen in children.
Hernia The most common cause of SBO in patients with no history of prior abdominal surgery is a hernia. A careful search for inguinal, femoral, and umbilical hernias must be made during evaluation of every patient presenting with symptoms consistent with obstruction. Internal hernias into the obturator foramen, the foramen of Winslow, or other anatomic defects must also be considered. In patients who have undergone previous surgery, incisional hernias represent another potential cause of SBO especially after laparotomy, in overweight or obese patients, in patients on steroid therapy or with wound infections.
Neoplasms Intrinsic small bowel neoplasms can progressively occlude the lumen or serve as a leading point in intussusception. Symptoms may be intermittent, onset of obstruction is slow, and signs of chronic anemia may be present. Peritoneal carcinomatosis from several tumors is an extrinsic cause of SBO due to adhesions of small bowel loops to neoplastic nodules.
Other causes of SBO include Crohn disease (CD), intussusception which is most often seen in children without an organic lesion and rarely in adults with a neoplastic intraluminal lesion; volvulus as a consequence of intestinal malrotation in children, or of adhesions in adults; and foreign bodies including bezoars, ingested foreign bodies, and gallstones through a cholecysto-duodenal fistula. Gallstone ileus is discussed in Chapter 25.
With the onset of obstruction, gas and fluid accumulate and distend the intestinal loops proximal to the site of obstruction. Fluid from the extracellular space also fills the lumen proximal to the obstruction, due to the impaired bidirectional flow of salt and water and fluid secretion enhanced by substances (endotoxins, prostaglandins) released from proliferating bacteria in the intestinal lumen. As a consequence, intraluminal and intramural pressures rise until microvascular perfusion to the intestine is impaired, leading to intestinal wall ischemia, and ultimately necrosis.
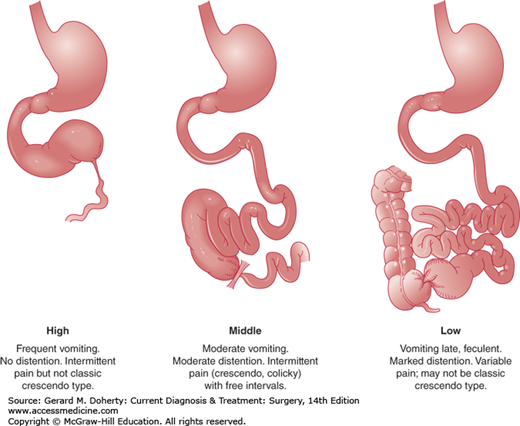
Activity of the smooth muscle of the small bowel is increased in an attempt to propel its contents past the obstruction consuming all energy sources. At this point the intestine becomes atonic and enlarges further. Emesis could be feculent due to bacterial overgrowth—particularly with distal obstruction—as the intestinal dilation progresses proximally (Figure 29–3). Bacterial translocation from the lumen to the mesenteric nodes and the bloodstream occurs and abdominal distention elevates the diaphragm and impairs respiration resulting in potential pulmonary complications such as pneumonia, and atelectasis.
When full thickness necrosis of the intestinal wall occurs, luminal content with an elevated bacterial load enters the peritoneal cavity, is absorbed by the peritoneum causing septic shock.
The progression of pathophysiologic events when the bowel is strangulated occurs more rapidly than with simple obstruction and is characterized by an acute impairment of venous return initially followed by arterial flow with subsequent ischemia, necrosis, and perforation of the intestinal wall.
Diagnostic evaluation should distinguish mechanical bowel obstruction from ileus, determine the cause of the obstruction, and recognize simple from strangulating obstruction.
Accurate diagnosis requires obtaining a detailed history paying particular attention to medications known to affect intestinal physiology, previous cancer, inflammatory bowel disease, and abdominal surgery and a meticulous physical examination.
Patients usually present with nausea, vomiting, colicky abdominal pain, and obstipation, although residual gas and stool distal to the obstruction may be expelled. With proximal SBO, emesis is usually profuse, containing undigested food in close temporal association with oral intake; abdominal pain is more often described as upper abdominal discomfort associated with epigastric distension. Distal SBO is characterized by diffuse and poorly localized crampy abdominal pain. Feculent vomiting present in cases of longstanding distal SBO is the consequence of bacterial overgrowth and is pathognomonic for a complete mechanical obstruction. In the presence of strangulation, fever often develops, and previously crampy abdominal pain becomes peritonitis.
Initially, vital signs may be normal, but tachycardia and hypotension usually develop as a result of progressive dehydration. Fever is often present with bowel ischemia or perforation. Inspection of the abdomen usually reveals distension that varies based on the site of obstruction and may be absent in cases of proximal obstruction. Peristalsis is usually tremendously increased in the early phases of mechanical SBO, as a result of intensive intestinal muscular contractions. This so-called “peristaltic rush” progressively decreases until it disappears in the late phase of obstruction. The presence of either surgical scars or hernias should be noted, indicating a possible cause of SBO. Rectal examination is essential to detect rectal lesions and to check for the presence of stool.
Laboratory findings reflect intravascular volume depletion and dehydration. An elevated hematocrit is indicative of hemoconcentration. Leukocytosis is often the result of dehydration and an acute stress response rather than an underlying infection. Blood chemistries may reveal elevated serum creatinine levels, indicating hypovolemia with prerenal failure.
Features of strangulated obstruction or perforation include marked leukocytosis and metabolic acidosis.
Plain x-rays of the abdomen with the patient in supine and upright position can confirm the clinical diagnosis of SBO. They reveal dilated small bowel loops with air-fluid levels in a ladder-like appearance, and a paucity of air in the colon. These features may be minimal or absent in early or high grade obstructions.
Computed tomographic (CT) scan of the abdomen and pelvis with both intravenous and oral contrast is widely used. CT scan can visualize the specific location of the obstruction, showing a discrepancy in the caliber between distended proximal bowel loops and collapsed distal intestine. Moreover, CT scan can also reveal the etiology of SBO and demonstrate signs of strangulation including thickening of the bowel wall, air in the bowel wall or portal venous system, and poor uptake of intravenous contrast by the affected bowel wall. Ascites between dilated bowel loops and in the pelvis is often reported in both simple and strangulated obstruction. Intraperitoneal free air indicates perforation.
Pain in patients with paralytic ileus is usually not severe but is constant and diffuse, and the abdomen is often distended and mildly tender. If ileus has resulted from an acute intraperitoneal inflammatory process, there should be symptoms and signs of the primary disease as well as the ileus. Abdominal x-rays show the presence of gas in both the colon and in the small bowel.
A postoperative ileus may be caused by several factors, including drugs used for anesthesia and analgesia, and intraoperative manipulation of intestinal loops and the mesentery. Usually, it is temporary; but if it persists for more than 3-5 days, diagnostic evaluation to rule out mechanical causes of obstruction is mandatory.
Colonic obstruction is usually diagnosed by abdominal x-rays that show colonic dilation proximal to the obstructing lesion. In the presence of competent ileocecal valve, a closed loop obstruction occurs with an elevated risk of perforation of the colon. If the ileocecal valve is incompetent, the distal small bowel will be dilated, and patients will exhibit abdominal distension, nausea and vomiting.
Acute gastroenteritis, acute appendicitis, and acute pancreatitis can mimic simple intestinal obstruction, while acute mesenteric ischemia (AMI) must be considered in the differential of small bowel strangulation.
Intestinal pseudo-obstruction comprises a spectrum of specific disorders associated with irreversible intestinal dysmotility, in which there are symptoms and signs of intestinal obstruction without evidence for an obstructing lesion. Acute pseudo-obstruction of the colon carries the risk of cecal perforation and is discussed in Chapter 30. Chronic pseudo-obstruction affecting the small bowel with or without colonic involvement can be idiopathic, or secondary to several (sporadic and familial) visceral myopathies and neuropathies that affect intestinal smooth muscle, and the intra- and extraintestinal nervous system. Systemic disorders such as scleroderma, myxedema, lupus erythematosus, amyloidosis, drug abuse (phenothiazine ingestion), radiation injury, or progressive systemic sclerosis can be complicated by chronic intestinal pseudo-obstruction. In addition, cytomegalovirus and Epstein–Barr viral infections can cause chronic intestinal pseudo-obstruction.
The clinical manifestations of chronic intestinal pseudo-obstruction include recurrent episodes of vomiting, crampy abdominal pain, and abdominal distention. The diagnosis is suggested by clinical findings and medical history, and confirmed by radiologic and manometric studies. Diagnostic laparoscopic full-thickness biopsy of the small bowel may be required to establish the specific cause of the disease. Therapy focuses on palliation of symptoms and nutritional issues.
SBO is associated with a marked depletion of liquids caused by decreased oral intake, vomiting, and sequestration of fluid in the bowel lumen. Therefore, vigorous fluid resuscitation and correction of electrolyte disorders (hypochloremic, hypokalemic metabolic alkalosis) is mandatory. A urinary catheter should be placed to monitor urinary output. Gastrointestinal decompression with a nasogastric tube provides relief of symptoms, prevents further gas and fluid accumulation proximally, and decreases the risk of aspiration. Obstruction that occurs in the early postoperative period is usually partial and only rarely associated with strangulation. Therefore, a period of prolonged total parenteral nutrition and hydration is warranted. Patients who have undergone numerous abdominal operations should initially be conservatively treated with decompression, bowel rest, and serial abdominal exams in hopes of avoiding reentering a hostile abdomen. Patients with CD rarely present with a complete bowel obstruction and these patients often benefit from steroids or other immunosuppressive therapy. However, if signs suggestive of ischemia are detected surgery should be promptly undertaken.
Finally, management of patients with diffuse carcinomatosis is often challenging and in most of cases limited to conservative and palliative treatment.
The surgical procedure performed varies according to the etiology of the obstruction. However, regardless of the cause of obstruction all small bowel loops must be examined and nonviable segments resected. Criteria suggesting viability include normal pink color, presence of peristalsis, and arterial pulsation.
Laparoscopic adhesiolysis may be performed in carefully selected patients by surgeons skilled in this procedure. Generally, however, an open procedure is performed through an incision that is partly dictated by the location of scars from previous operations.
If the cause of the obstruction cannot be removed, as in case with infiltration of vital structures by cancer or in the case of diffuse carcinomatosis, an anastomosis between proximal small bowel and small or large bowel distal to the obstruction (bypass) may be the best procedure in these patients. In some cases, a stoma can be the only choice of treatment.
Vast majority (more than 80%) of patients with adhesive SBO do not need an operation, since they improve with medical therapy. Among patients who require surgery, perioperative mortality rate for nonstrangulating obstruction is less than 5%; most of these deaths occur in elderly patients with significant comorbidities. Strangulating obstruction has a mortality rate of approximately 8% if surgery is performed within 36 hours of the onset of symptoms and 25% if operation is delayed beyond 36 hours.
REGIONAL ENTERITIS
Crohn disease is a chronic inflammatory disease that commonly affects the small bowel, colon, rectum, and anus, but it can also involve the stomach, esophagus, and mouth. CD is a panintestinal condition which may affect any area from the mouth to the anus. The most commonly affected location is the terminal ileum and one-fifth of all patients have more than one intestinal segment affected simultaneously.
The United States, Canada, and Europe have the highest incidence of CD. The current estimated incidence of CD in the United States is approximately four new cases per year for every 100,000 persons, while the prevalence is much higher, between 80 and 150 cases per 100,000.
It is much less common in Asia, South America, and Japan, while accurate data regarding its incidence in Africa are lacking. The peak age for contracting CD is between 15 and 25 years. Familial clusters of disease are not uncommon, with a six- to tenfold increase in the risk of CD in first-degree relatives of those affected by CD or its sister ailment, ulcerative colitis. Although familial aggregations are common, the distribution within families does not indicate a pattern of simple Mendelian inheritance.
The etiology of CD is not known. CD is an altered immune response that results in inflammation and destruction of intestinal tissues. If this altered immune response is the result of a primary dysfunction in the gut-related immune system or whether an unknown pathological trigger induces an otherwise normal immune system to overreact is still unclear. CD may occur in individuals with a genetic predisposition, while environmental triggers may start the pathological sequence that ultimately manifests as CD.
To date, even though an increase in intestinal permeability in both CD patients and their symptom-free first-degree relatives has been demonstrated no specific primary defect in the systemic or mucosal immune system has been identified. This may lead to an altered mucosal barrier function with abnormal interactions between the multitude of antigenic substrates normally found in the gut lumen and the immunocompetent tissue of the submucosa.
Concerning genetic predisposition, the CARD15/NOD2 gene has been linked to susceptibility to CD. CARD15 is a gene product related to innate immunity and it is preferentially expressed to Paneth cells of the ileum. Nevertheless, the known mutations of CARD15 are neither necessary nor sufficient to contract the disease. Hence, it appears that the genetic relationship of CARD15/NOD2 to CD is complex and still poorly understood.
The hypothesis that infectious agents may play a role, either directly as a primary cause of CD, or indirectly as a trigger to stimulate a defective immune system, has always found strength in the identification of noncaseating granulomas as the characteristic histopathologic lesion found in Crohn specimens, and in the isolation of Mycobacterium paratuberculosis from resected CD specimens. Nevertheless, even sensitive preliminary chain reaction studies have been unable to provide definitive evidence for the presence of Mycobacterium paratuberculosis-specific DNA in CD-affected segments of the bowel. Other infectious agents, including measles virus, non-pylori Helicobacter species, Pseudomonas, and Listeria monocytogenes have been studied, but none of them has been consistently associated with CD.
Although diet modification can ameliorate the symptoms of CD, no dietary factor has been identified as a cause of CD. Smoking, however, has been associated with the development of CD. In addition, smoking is known to exacerbate existing CD and can accelerate the recurrence of disease after resection.
Histopathologic examination of CD typically demonstrates transmural inflammation characterized by multiple lymphoid aggregates in a thickened and edematous submucosa that can be found within the muscularis propria. Another typical microscopic feature of CD is the noncaseating granuloma. However, it is demonstrated in only 50% of resected specimens and is rarely detected on endoscopic biopsies. Additionally, the presence of granulomas does not correlate with disease activity.
Small mucosal ulcerations, called aphthous ulcers, are the earliest gross manifestations of CD. They appear as red spots or focal mucosal depressions, typically directly over submucosal lymphoid aggregates. As the inflammation progresses, the aphthous ulcers enlarge and become stellate. They then coalesce to form longitudinal mucosal ulcerations always along the mesenteric aspect of the bowel lumen. Further progression leads to a serpiginous network of linear ulcerations that surround islands of edematous mucosa producing the classic “cobblestone” appearance. Mucosal ulcerations may penetrate through the submucosa to form intramural channels that can bore deeply into the bowel wall and create sinuses, abscesses, or fistulas.
The inflammation of CD also involves the mesentery and regional lymph nodes such that the mesentery may become massively thickened. With early acute intestinal inflammation, the bowel wall is hyperemic and boggy. As the inflammation becomes chronic, fibrotic scarring develops and the bowel wall becomes thickened and leathery in texture.
The clinical presentation and symptoms of CD depend on the involved segment, the pattern and the severity of disease, and the associated complications. The onset of CD is often insidious and many patients will experience some symptoms for months or even years before the diagnosis is made. The most common complaints are intermittent abdominal pain, bloating, diarrhea, nausea, vomiting, weight loss, and fever. Abdominal pain occurs in 90% of cases: when related to partial obstruction it is mostly postprandial and crampy in nature, while when it is from septic complications it is typically steady and associated with fever. Weight loss is usually related to food avoidance, but in severe cases weight loss may be the result of malabsorption. Symptoms can also be related to complications including abdominal mass, pneumaturia, perianal pain and swelling, or skin rash. Rarely some patients can experience a more sudden onset of pain in the right-lower quadrant, mimicking an acute appendicitis.
In patients suspected of having CD, a complete physical exam should include a thorough abdominal evaluation. In cases of ileal CD, tenderness is typically present in the right-lower quadrant and occasionally a palpable mass is present. The oral cavity should be examined for aphthous ulcers, while the presence of fistulas, abscesses, or enlarged skin tags should be assessed in the perianal area. A digital rectal examination should assess for the presence of anal strictures, fissures, and rectal mucosal ulcerations. The skin in the extremities should be examined for the presence of erythema nodosum and pyoderma gangrenosum.
Even though CD can be categorized into three general manifestations, such as stricturing, perforating, and inflammatory disease, these three classes do not represent truly distinct forms of the disease. It is typical that the same patient can present with more than one pattern even in the same segment of bowel. Nevertheless, one pattern tends to be predominant in most cases, determining the clinical presentation and affecting the therapeutic options.
Fibrotic scar tissue is the result of chronic inflammation of CD, and it constricts the intestinal lumen with cicatricial strictures often referred to as “fibrostenotic lesions.” Patients with a stricturing pattern of disease generally develop partial or complete intestinal obstruction, and hence their symptoms are primarily obstructive in nature. Being the result of scar tissue, these strictures are not reversible with medical therapy and surgical intervention is often required.
Perforating CD is characterized by the development of sinus tracts, fistulae, and abscesses. The sinus tracts penetrate through the muscularis propria and give rise to abscesses or to fistulas if they penetrate into surrounding structures. Inflammatory response around the advancing sinus tract typically results in adhesion to surrounding structures, therefore, free perforation with spillage of intestinal contents into the abdominal cavity is uncommon. Typically, perforating disease is accompanied by a degree of stricture formation, but the fistula or abscess generated by the perforating component of the disease dominates the clinical picture.
The inflammatory pattern of CD is characterized by mucosal ulceration and bowel wall thickening. The edema that results from inflammation can lead to an adynamic segment of intestine and luminal narrowing. This pattern often gives rise to obstructive symptoms. Of the three patterns of disease, the inflammatory pattern is much more likely to respond to medical therapy.
Other common symptoms and findings include anorexia and weight loss. Patients may develop a palpable mass, usually located in the right-lower quadrant, related to an abscess or phlegmon in perforating disease or a thickened loop of intestine in obstructive disease. Evidence of fistulization to the skin, urinary bladder, or vagina may also be elicited with an accurate history and physical exam.
There is no specific laboratory test that is diagnostic for CD. The diagnosis is made by a thorough history and physical examination along with intestinal radiography and endoscopy. Advanced imaging studies such as CT scan or magnetic resonance imaging (MRI) can assess or detect some of the complications and manifestations of CD, but they are generally not useful in making the initial diagnosis of CD.
Small bowel follow-through or enteroclysis are the best means for assessing the small bowel for CD. The radiographic abnormalities are often distinctive. Mucosal granulations with ulceration and nodularity can be identified in the early stages of the disease. Thickening of the mucosal folds and edema of the bowel wall can be demonstrated as the disease progresses. With more advanced disease, cobblestoning becomes radiographically apparent. Small bowel contrast studies can also provide information regarding enlargement of the mesentery, as well as formation of an inflammatory mass or abscess demonstrated by a general mass effect separating and displacing contrast-filled loops of small intestine. Even though small bowel contrast studies can demonstrate some of the complications of CD, including high-grade strictures and fistulas, they may not identify all such lesions, including ileosigmoid and ileovesical fistulas. Additionally, small bowel studies may not demonstrate all the areas of disease with significant strictures. Small bowel radiographs can also help in assessing the extent of the disease by identifying the location and length of involved and uninvolved small bowel, and by recognizing whether the disease is continuous or discontinuous with skip lesions separated by areas of normal intestine. Experienced radiologists can also assess areas of luminal narrowing and determine if they are the result of acute inflammatory swelling or are the result of fibrostenotic scar tissue. Such a distinction provides valuable information regarding the value of medical therapy versus early surgical intervention, as inflammatory stenoses are likely to respond to medical therapy while fibrotic strictures are best treated with surgery.
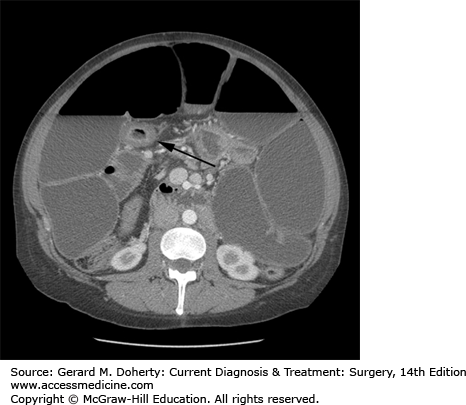
Computed tomography findings of uncomplicated CD are nonspecific and routine CT is not necessary for the diagnosis of CD. CT, however, is very useful in identifying the complications associated with CD—thickened and dilated intestinal loops, inflammatory masses, abscesses, and hydronephrosis resulting from retroperitoneal fibrosis and ureteral narrowing (Figure 29–4). CT is also the most sensitive indicator of an enterovesical fistula as suggested by the presence of air within the urinary bladder. More recently cross-sectional imaging techniques have assumed an increasing role in the imaging of patients with CD. Computed tomography enterography (CTE) has been shown to have a higher sensitivity than barium small-bowel follow through. Based on these findings, CTE is often used combined with ileocolonoscopy as a first-line test for the diagnosis and staging of CD. CTE has several potential advantages over barium studies in the identification of fistulizing disease. CTE does not suffer from superimposition of bowel loops, and it displays the mesentery, retroperitoneum, and abdominal wall musculature, typically involved by fistulas. CTE can also readily identify sinus tracts and abscesses. However, recent concerns about radiation-induced cancer arising from medically related CT in young CD patients have encouraged the use of magnetic resonance enterography (MRE). MRE has the same advantages of CTE but does not require ionizing radiation.
While upper endoscopy is useful in the diagnosis of mucosal lesions of the esophagus, stomach, and duodenum a colonoscopy often allows the evaluation of the terminal ileum.
Capsule endoscopy can detect subtle mucosal lesions that may not be apparent on small bowel x-rays. The value of capsule endoscopy in the diagnosis of CD has been recently evaluated: the rate of abnormalities detected on capsule endoscopy is higher than that of CTE only for the subgroup of patients with known CD. The need for a preliminary small bowel contrast study to detect asymptomatic partial small-bowel obstruction before the capsule endoscopy and the lack of a clear advantage over other imaging studies, limits the utility of capsule endoscopy as a first-line test in CD, and perhaps reserve this study for those cases in which there is a substantial diagnostic uncertainty.
The differential diagnosis includes irritable bowel syndrome, acute appendicitis, intestinal ischemia, pelvic inflammatory disease, endometriosis, and gynecological malignancies. Other disorders are radiation enteritis, Yersinia infections, intestinal injury from nonsteroidal anti-inflammatory agents, intestinal tuberculosis, and small bowel tumors.
When malignancy is suspected, resection should be undertaken to make the diagnosis certain. The exclusion of intestinal tuberculosis can be difficult, as the inflammation and strictures of the terminal ileum can occur very similarly to CD. A previous exposure to tuberculosis should be evaluated and purified protein derivative skin test should be performed, along with chest radiography. Even when the diagnosis of CD is certain, patients who coincidentally are found to also have latent tuberculosis should be treated in accordance with the American Thoracic Society guidelines prior to the initiation of immunosuppressive therapy for management of CD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree