Sleep Disorders
KEY CONCEPTS
![]() Common causes of insomnia include concomitant psychiatric disorders, significant psychosocial stressors, excessive alcohol use, caffeine intake, and nicotine use.
Common causes of insomnia include concomitant psychiatric disorders, significant psychosocial stressors, excessive alcohol use, caffeine intake, and nicotine use.
![]() Good sleep hygiene, including relaxing before bedtime, exercising regularly, establishing a regular bedtime and wake-up time, and discontinuing alcohol, caffeine, and nicotine, alone and in combination with drug therapy, should be part of patient education and treatments for insomnia.
Good sleep hygiene, including relaxing before bedtime, exercising regularly, establishing a regular bedtime and wake-up time, and discontinuing alcohol, caffeine, and nicotine, alone and in combination with drug therapy, should be part of patient education and treatments for insomnia.
![]() Long-acting benzodiazepines should be avoided in the elderly.
Long-acting benzodiazepines should be avoided in the elderly.
![]() Benzodiazepine tolerance and dependence are avoided by using low-dose therapy for the shortest possible duration.
Benzodiazepine tolerance and dependence are avoided by using low-dose therapy for the shortest possible duration.
![]() Obstructive sleep apnea may be an independent risk factor for the development of hypertension. When hypertension is present, it is often refractory to drug therapy until sleep-disordered breathing is alleviated.
Obstructive sleep apnea may be an independent risk factor for the development of hypertension. When hypertension is present, it is often refractory to drug therapy until sleep-disordered breathing is alleviated.
![]() Nasal continuous positive airway pressure is the first-line therapy for obstructive sleep apnea, and weight loss should be encouraged in all obese patients.
Nasal continuous positive airway pressure is the first-line therapy for obstructive sleep apnea, and weight loss should be encouraged in all obese patients.
![]() Pharmacologic management of narcolepsy is focused on two primary areas: treatment of excessive daytime sleepiness and rapid eye movement (REM) sleep abnormalities.
Pharmacologic management of narcolepsy is focused on two primary areas: treatment of excessive daytime sleepiness and rapid eye movement (REM) sleep abnormalities.
![]() Short-acting benzodiazepine receptor agonists, ramelteon, or melatonin taken at appropriate target bedtimes for east or west travel reduce jet lag and shorten sleep latency.
Short-acting benzodiazepine receptor agonists, ramelteon, or melatonin taken at appropriate target bedtimes for east or west travel reduce jet lag and shorten sleep latency.
![]() Dopamine agonists are effective for restless legs syndrome and have replaced levodopa as first-line therapy.
Dopamine agonists are effective for restless legs syndrome and have replaced levodopa as first-line therapy.
Approximately 70 million Americans suffer with a sleep-related problem, and as many as 60% of those experience a chronic disorder.1 In a study by the National Institute on Aging, of 9,000 patients aged 65 years and older, more than 80% report a sleep-related disturbance.1
INTRODUCTION TO SLEEP
Sleep Cycles
Sleep is divided into two phases: nonrapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. Each night humans typically experience four to six cycles of NREM and REM sleep, with each cycle lasting between 70 and 120 minutes.2 There are four stages of NREM sleep. Healthy sleep will typically progress through the four stages of NREM sleep prior to the first REM period. From wakefulness, sleep typically progresses quickly through stages 1 and 2. Stage 1 of NREM sleep is the stage between wakefulness and sleep, and individuals describe this experience as being awake, being drowsy, or being asleep. During stages 3 and 4 NREM, both metabolic activity and brain waves slow. This slow-wave sleep occurs most frequently early in the sleep period. Stages 3 and 4 sleep are called delta sleep, as the sleep is characterized by high-amplitude slow activity known as delta waves (0.5 to 3 Hz) with no eye movements and low tonic muscle activity.
REM sleep involves a dramatic physiologic change from NREM sleep, to a state in which the brain becomes electrically and metabolically activated.2 REM occurs in bursts and is accompanied by a 62% to 173% increase in cerebral blood flow, generalized muscle atonia, bursts of bilateral REMs, poikilothermia, dreaming, and fluctuations in respiratory and cardiac rate.2 REM cycles tend to lengthen in the later stages of the sleep cycle.2
Circadian Rhythm
At birth human infants spend up to 20 hours a day sleeping. At 3 to 6 months of age there is a differentiation between REM and NREM sleep. By age 3 years the ultradian sleep–wake rhythm changes to a circadian pattern. The suprachiasmatic nucleus of the brain serves as the biologic clock and paces the circadian rhythm. Although the length of a day is 24 hours, in environments devoid of light cues, the sleep–wake cycle lasts about 25 hours.3 In midlife, there is a gradual decline in sleep efficiency and sleep time.2 The elderly have lighter and more fragmented sleep, with intermittent arousals, shifts in the sleep stages, and a gradual reduction of slow-wave sleep.
Neurochemistry
The neurochemistry of sleep is complex, as sleep cannot be localized to either a specific area of the brain or a neurotransmitter. NREM sleep appears to be controlled by the basal forebrain, the lower brain stem to the thalamus, and hypothalamus.3 Numerous neurotransmitters mediate NREM sleep, including γ-aminobutyric acid (GABA) and adenosine.3 REM sleep appears to be turned on by cholinergic cells in the mesencephalic, medullary, and pontine gigantocellular regions. REM sleep appears to be turned off by the dorsal raphe nucleus, the locus coeruleus, and the nucleus para-brachialis lateralis, the latter two of which are primarily noradrenergic. The ascending reticular activating system and the posterior hypothalamus facilitate arousal and wakefulness.4 Dopamine has an alerting effect; decreases in dopamine promote sleepiness.5 Neurochemicals involved in wakefulness include norepinephrine and acetylcholine in the cortex and histamine and neuropeptides such as substance P and corticotropin-releasing factor in the hypothalamus.5,6
Polysomnography
Sleep is typically measured and observed in sleep laboratories using an electroencephalogram (EEG), electrooculograms of each eye, electrocardiogram, electromyogram, air thermistors, abdominal and thoracic strain belts, and oxygen saturation monitor. This study is named polysomnography (PSG) and is used to assess and record variables that characterize sleep and aid in diagnosis of sleep disorders. Variables obtained during PSG include sleep onset, arousals, sleep stages, eye movements, leg and jaw movements, arrhythmias, airflow during sleep, respiratory effort, and oxygen desaturations. Home sleep monitoring that measures variables such as electrocardiogram, oxygen saturation, airflow, and respiratory effort is also increasingly used to diagnose sleep apnea.
CLASSIFICATION OF SLEEP DISORDERS
The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) classifies sleep disorders into four categories based on etiology and requires a symptom duration of at least 1 month before a sleep disorder can be diagnosed.7,8 Primary sleep disorders are those disorders in which there is no other etiology (mental disorder, substance-related disorder, or medical condition) responsible for the disorder. They appear to be based on an endogenous abnormality of the sleep–wake cycle or circadian rhythm, and they are divided into dyssomnias (abnormality in the amount, quality, or timing of sleep) and parasomnias (abnormal behavioral or physiologic events associated with sleep, e.g., sleepwalking and REM behavior disorder). Dyssomnias include sleep disorders such as insomnia, narcolepsy, obstructive sleep apnea (OSA), and circadian rhythm disorders.
Insomnia
Insomnia is the most common complaint in general medical practice.9 It causes distress, frequently because of a fear or a feeling of not being able to fall asleep at bedtime, and can impair work-related productivity because of daytime fatigue or drowsiness. Insomnia is subjectively characterized as a complaint of difficulty falling asleep, difficulty maintaining sleep, or experiencing nonrestorative sleep.7,8 Insomnia lasting two or three nights is considered to be transient insomnia, whereas short-term insomnia usually resolves in less than 3 weeks. Insomnia, according to the DSM-IV-TR, is considered to be chronic when it lasts longer than 1 month.8
Epidemiology
Primary insomnia usually begins in early or middle adulthood and is rare in childhood or adolescence. Symptoms of insomnia occur in 33% to 50% of the adult population.9 A 1-year prevalence study of insomnia in the United States reports that one third of the individuals surveyed complained of insomnia, and 17% reported that the symptoms were serious.1 Conservative estimates of chronic insomnia range from 9% to 12% in adulthood and up to 20% in the elderly.1,10 Although young adults are more likely to complain that they have difficulty falling asleep, middle-aged and elderly adults are more likely to complain that they have middle-of-the-night awakening or early morning awakening. Women complain of insomnia twice as frequently as men. Individuals who are elderly, unemployed, separated, or widowed, and those with a lower socioeconomic status report a significantly higher incidence of insomnia than the general population. Forty percent of individuals with insomnia also have a concurrent psychiatric disorder (anxiety, depression, or substance abuse).11
Approximately 10% to 20% of those with insomnia use nonprescription drugs or alcohol to self-treat.
Differential Diagnosis
Primary insomnia is considered to be an endogenous disorder caused by either a neurochemical or a structural disorder affecting the sleep–wake cycle. Individuals with primary insomnia can be light sleepers who are easily aroused by noise, temperature, or anxiety. Some studies suggest that primary insomnia is a “hyperarousal state,” in that insomnia patients have increased metabolic rates compared with controls and thus take longer to fall asleep.2 Comorbid or secondary insomnia is frequently a symptom or manifestation of another medical disorder. Evaluation of patients with a complaint of transient or short-term insomnia should focus on recent stressors, such as a separation, a death in the family, a job change, or college exams.
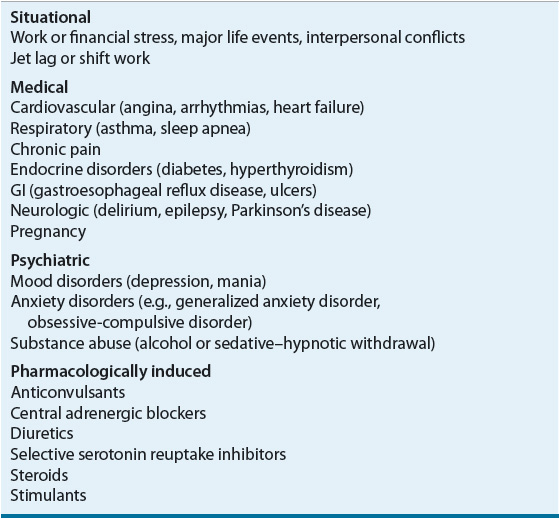
![]() Chronic insomnia is frequently comorbid with psychiatric or medical conditions. A complete diagnostic examination should be completed in these individuals and should include routine laboratory tests, physical and mental status examinations, as well as ruling out any medication- or substance-related causes.12 Special consideration should also be given to other sleep disorders that can have a similar presentation, including restless legs syndrome (RLS), periodic limb movements of sleep (PLMS), and sleep apnea. Common causes of insomnia are listed in Table 55-1.
Chronic insomnia is frequently comorbid with psychiatric or medical conditions. A complete diagnostic examination should be completed in these individuals and should include routine laboratory tests, physical and mental status examinations, as well as ruling out any medication- or substance-related causes.12 Special consideration should also be given to other sleep disorders that can have a similar presentation, including restless legs syndrome (RLS), periodic limb movements of sleep (PLMS), and sleep apnea. Common causes of insomnia are listed in Table 55-1.
TABLE 55-1 Common Etiologies of Insomnia
TREATMENT
Desired Outcomes
The goals of treatment of insomnia are to correct the underlying sleep complaint, consolidate sleep, improve daytime functioning and sleepiness, and avoid adverse effects from selected therapies. Drug therapy should be used in the lowest possible dose, for the shortest possible time period.
Treatment Principles
Therapeutic management of insomnia is initially based on whether the individual has experienced a transient, short-term, or chronic sleep disturbance. Clinical history should assess the onset, duration, and frequency of the symptoms; effect on daytime functioning; sleep hygiene habits; and history of previous symptoms or treatment.13 Management of all patients with insomnia should include identifying the cause of the insomnia, patient education on sleep hygiene, and stress management. Any unnecessary pharmacotherapy should be eliminated.10 Transient insomnia, which occurs as a result of an acute stressor, is expected to resolve quickly and should be treated with good sleep hygiene and careful use of sedative–hypnotics.11 Short-term insomnia, associated with situational, personal, or medical stress, can be treated similarly.13 Chronic insomnia requires careful assessment for possible underlying medical causes, nonpharmacologic approaches, and careful use of sedative–hypnotics.12
Nonpharmacologic Therapy
![]() In many cases insomnia can be treated without sedative–hypnotics. Education about normal sleep and habits for good sleep hygiene are important for all patients with insomnia. Nonpharmacologic interventions for insomnia frequently consist of short-term cognitive behavioral therapies, most commonly stimulus control therapy, sleep restriction, relaxation therapy, cognitive therapy, paradoxical intention, biofeedback, and education on good sleep hygiene (Table 55-2).10,14 In patients aged 55 and older, research indicates that cognitive behavioral therapy may be more effective than pharmacologic therapy at improving certain measures of insomnia.15,16
In many cases insomnia can be treated without sedative–hypnotics. Education about normal sleep and habits for good sleep hygiene are important for all patients with insomnia. Nonpharmacologic interventions for insomnia frequently consist of short-term cognitive behavioral therapies, most commonly stimulus control therapy, sleep restriction, relaxation therapy, cognitive therapy, paradoxical intention, biofeedback, and education on good sleep hygiene (Table 55-2).10,14 In patients aged 55 and older, research indicates that cognitive behavioral therapy may be more effective than pharmacologic therapy at improving certain measures of insomnia.15,16
TABLE 55-2 Nonpharmacologic Recommendations for Management of Insomnia

Pharmacologic Therapy
Miscellaneous Agents
Antihistamines exhibit sedating properties and are included in many nonprescription sleep agents. They are effective in the treatment of mild insomnia and are generally safe.13 Diphenhydramine and doxylamine are more sedating than pyrilamine. Patients quickly experience tolerance to sedative effects, and increasing the dose of antihistamines will not produce a linear increase in response. Antihistamines are considered to be less effective than benzodiazepines, and they have the disadvantages of anticholinergic side effects, which are especially troublesome in the elderly.13,17
Antidepressants are alternatives for patients with nonrestorative sleep who should not receive benzodiazepines, especially those who have depression, pain, or a risk of substance abuse. Using antidepressants for insomnia without depression is common but not well studied, and the doses used for treating insomnia are not effective antidepressant doses.9,13,14 Sedating antidepressants such as amitriptyline, doxepin, and nortriptyline are effective for inducing sleep continuity, although daytime sedation and side effects can be significant.9,13 Anticholinergic activity, adrenergic blockade, and cardiac conduction prolongation can be problematic, especially in the elderly and in overdose situations.9 Low-dose doxepin (3 to 6 mg) was recently FDA-approved for the treatment of sleep maintenance insomnia. Mirtazapine is a sedating antidepressant that may help patients sleep, but it may also cause daytime sedation and weight gain.
Trazodone in doses of 25 to 100 mg at bedtime is sedating and can improve sleep continuity.11 Trazodone is popular for the treatment of insomnia in patients prone to substance abuse, as dependence is not a problem with trazodone, and in patients with selective serotonin reuptake inhibitor and bupropion-induced insomnia.11 Other side effects include carryover sedation and α-adrenergic blockade. Orthostasis can occur at any age, but it is more dangerous in the elderly. Priapism is a rare but serious side effect.18
Ramelteon is a melatonin receptor agonist approved for the treatment of sleep-onset insomnia. It is selective for the MT1 and MT2 melatonin receptors that are thought to regulate the circadian rhythm and sleep onset. The recommended dose is 8 mg taken at bedtime to induce sleep. Although generally well tolerated, the most common adverse events reported are headache, dizziness, and somnolence. Ramelteon is not a controlled substance and can be a viable option for patients with a history of substance abuse. It effectively treats sleep-onset difficulties in patients with chronic obstructive pulmonary disease and sleep apnea.19,20
Valerian is a herbal sleep remedy that has been studied for its sedative–hypnotic properties in patients with insomnia. The mechanism of action is not fully understood but may involve increasing concentrations of GABA. The recommended dose for insomnia ranges from 300 to 600 mg. An equivalent dose of dried herbal valerian root is 2 to 3 g soaked in one cup of hot water for 20 to 25 minutes.21
Benzodiazepine Receptor Agonists
The most commonly used treatments for insomnia have been the benzodiazepine receptor agonists (BZDRAs). BZDRAs are effective as sedative–hypnotics and are FDA-labeled for the treatment of insomnia (Table 55-3). The FDA requires BZDRA labeling to include a caution regarding anaphylaxis, facial angioedema, and complex sleep behaviors (e.g., sleep driving, phone calls, sleep eating, etc.). The BZDRAs consist of the newer nonbenzodiazepine GABAA agonists and the traditional benzodiazepines. All BZDRAs bind to GABAA receptors in the brain, resulting in agonist effects on GABAergic transmission and hyperpolarization of neuronal membranes. Traditional benzodiazepines have sedative, anxiolytic, muscle relaxant, and anticonvulsant properties; newer nonbenzodiazepine GABA agonists possess only sedative properties.
TABLE 55-3 Pharmacokinetics of Benzodiazepine Receptor Agonists
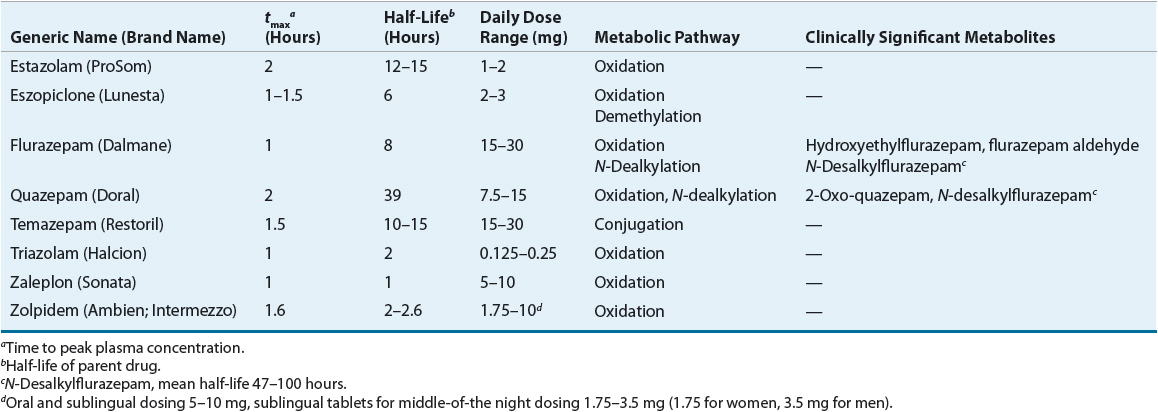
Benzodiazepine Hypnotics
Benzodiazepines relieve insomnia by reducing sleep latency and increasing total sleep time. They increase stage 2 sleep while decreasing delta sleep.11 Benzodiazepine hypnotics should not be prescribed for individuals who are pregnant or who have untreated sleep apnea or a history of substance abuse. Patients should be instructed to avoid alcohol and other CNS depressants.
Adverse Effects Side effects are dose dependent and vary according to the pharmacokinetics of the individual benzodiazepine. High doses with long or intermediate elimination half-lives have a greater potential for producing daytime sedation, psychomotor incoordination, and cognitive deficits. Most traditional benzodiazepines maintain hypnotic efficacy for 1 month. However, tolerance can develop with time.
Anterograde amnesia, an impairment of memory and recall of events occurring after the dose is taken, has been reported with most BZDRAs (it is more likely to occur with short-acting agents).11 Rebound insomnia, characterized by increased wakefulness beyond baseline amounts that last for one to two nights after abrupt discontinuation, occurs with BZDRAs. The lowest effective dosage should be used to minimize rebound insomnia and avoid adverse effects on memory.
![]() Benzodiazepine half-lives are prolonged in older patients, increasing the potential for drug accumulation and the incidence of CNS side effects, including prolonged sedation and cognitive and psychomotor impairment. BZDRAs with long elimination half-lives (e.g., flurazepam and quazepam) are generally not first-line agents in these patients. Benzodiazepine use is associated with increased risk of falls and hip fractures in the elderly, but since insomnia itself increases fall and fracture risk, it is unclear if benzodiazepines increase risk independent of sleep problems.22
Benzodiazepine half-lives are prolonged in older patients, increasing the potential for drug accumulation and the incidence of CNS side effects, including prolonged sedation and cognitive and psychomotor impairment. BZDRAs with long elimination half-lives (e.g., flurazepam and quazepam) are generally not first-line agents in these patients. Benzodiazepine use is associated with increased risk of falls and hip fractures in the elderly, but since insomnia itself increases fall and fracture risk, it is unclear if benzodiazepines increase risk independent of sleep problems.22
Clinical Controversy…
Nonbenzodiazepine GABAA Agonists
Zolpidem, zaleplon, and eszopiclone are nonbenzodiazepine hypnotics that selectively bind to GABAA receptors and effectively induce sleepiness. Zolpidem has a duration of action of 6 to 8 hours.23 It is comparable in efficacy to benzodiazepine hypnotics and is effective for reducing sleep latency and nocturnal awakenings and increasing total sleep time. It does not appear to have significant effects on next-day psychomotor performance. Sustained-release, sublingual, and reduced-strength (1.75 and 3.5 mg) formulations of zolpidem are available and are used to increase total sleep time, to reduce sleep latency, and for middle-of-the night rescue dosing, respectively.
Zolpidem is less disruptive of sleep stages than benzodiazepines. Adverse effects are dose related and can include drowsiness, amnesia, dizziness, headache, and GI complaints.23 Sleep eating during zolpidem therapy can result in significant weight gain.23 The recommended daily dose of zolpidem is 10 or 5 mg in elderly patients and those with hepatic impairment. Because food decreases its absorption, zolpidem should be taken on an empty stomach.24
Zaleplon has a rapid onset of action and a half-life of 1 hour, and it is metabolized to inactive metabolites.25 It is effective for decreasing time to sleep onset but not for reducing nighttime awakening or for increasing total sleep time.26 Because of its short half-life, zaleplon has no effect on next-day psychomotor performance and can be used as a sleep aid for middle-of-the-night awakenings.27 The recommended dose is 10 mg in adults and 5 mg in the elderly.25 The most common adverse effects with zaleplon are dizziness, headache, and somnolence. There are two drug interactions of note: zaleplon plasma levels are increased when combined with cimetidine and decreased with rifampin.23
Eszopiclone is effective at reducing time to sleep onset, wake time after sleep onset, and number of awakenings, and increasing total sleep time and sleep quality. Eszopiclone’s duration of action is up to 6 hours,28 so it can be a good option for treatment of sleep maintenance insomnia or early morning awakenings. The most common adverse effects with eszopiclone are somnolence, unpleasant taste, headache, and dry mouth.28 Eszopiclone is labeled for long-term use and may be taken nightly for up to 6 months.28,29
Other Considerations
In general, the nonbenzodiazepine hypnotics seem to be associated with less withdrawal, tolerance, and rebound insomnia than the benzodiazepine hypnotics. None of the nonbenzodiazepine GABAA agonists have significant active metabolites.
Evaluation of Therapeutic Outcomes
An algorithm for the evaluation and treatment of dyssomnias is shown in Figure 55-1. Patients with short-term or chronic insomnia should be evaluated after 1 week of therapy to assess for drug efficacy, adverse effects, and adherence to nonpharmacologic recommendations.
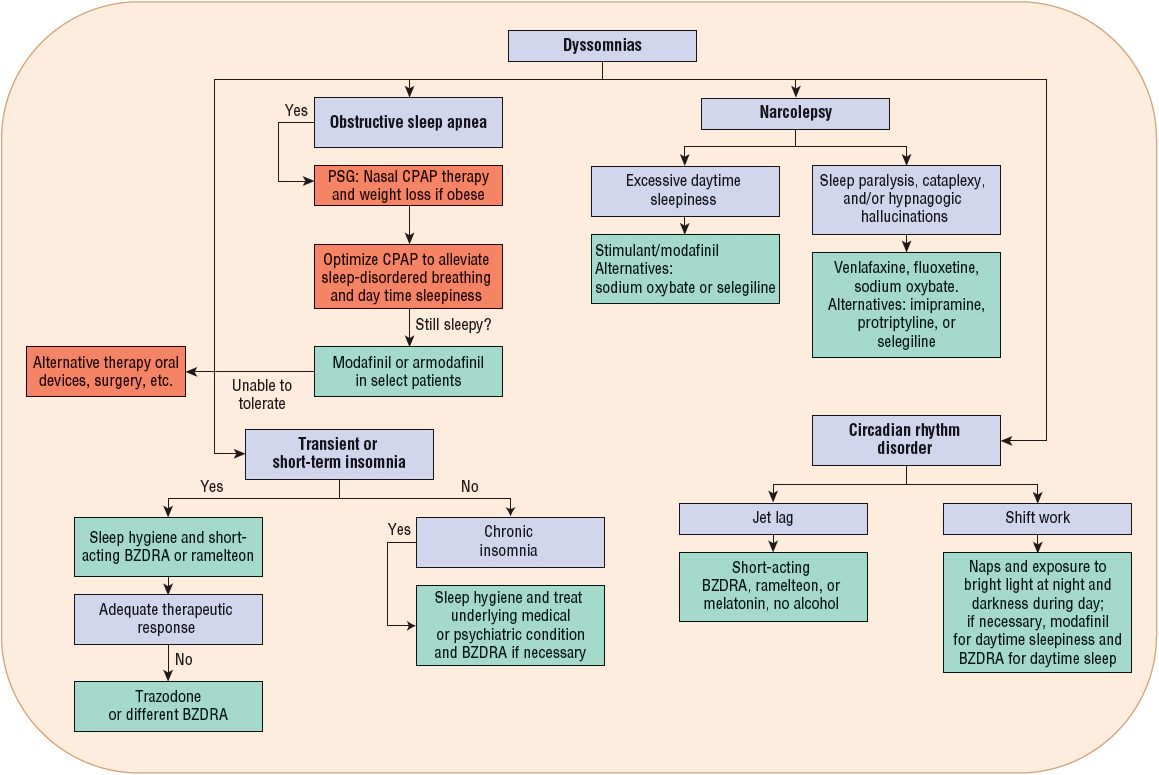
FIGURE 55-1 Algorithm for treatment of dyssomnias. (BZDRA, benzodiazepine receptor agonist; CPAP, continuous positive airway pressure.) (Adapted with permission from reference 30.)



