Skin: Nonneoplastic Dermatopathology
Samuel J. Pruden II
Kimberley G. Crone
Anne C. Lind
I. NORMAL MICROANATOMY. Microscopically, the skin is composed of three compartments: the epidermis (a keratinizing epithelium), the dermis (a connective tissue matrix), and the subcutis (a layer of adipose tissue). Specific features and the relative size of each of these compartments vary with body site and age and reflect the many functions of the skin. Familiarity with regional anatomical differences helps recognition of subtle abnormalities, provides a clue to the site of a biopsy if that information is not provided, and aids in the formulation of a differential diagnosis that is appropriate to specific anatomic locations.
The epidermis, a stratified squamous epithelium, rests on a normally invisible basement membrane. The keratinocytes of the basal layer (stratum basale) have a generative function and are anchored to the basement membrane by hemidesmosomes. Immediately above the basal layer is the variably thick “prickle” or “spinous” cell layer (stratum spinosum). This name refers to the slender eosinophilic processes that extend between adjacent keratinocytes as seen by light microscopy. These processes correspond to the desmosomes or cytoplasmic attachment plaques. Above the spinous layer is the granular layer (stratum granulosum), which features fine intracytoplasmic basophilic keratohyaline granules. The granular cell layer is 1 to 3 cells thick and forms a water-tight barrier. The most mature and outermost cell layer of the epidermis, the cornified layer (stratum corneum), is composed of flat keratinocytes without nuclei. Keratinocytes are derived from ectoderm and produce type I small acidic (K9-20) and type II large neutral-to-basic (K1-8) keratins. Transit time through all layers of the epidermis is ˜28 days.
Cells other than keratinocytes are also present in the epidermis. Melanocytes originate in the neural crest and are normally located in the basal layer slightly beneath the basal keratinocytes. They are histologically distinct, having a rounded, hyperchromatic nucleus as compared with the more elongated nucleus of the basal keratinocyte. They sometimes show a pericytoplasmic clearing/vacuole. This is an artifact of fixation and is attributable to their lack of desmosomes. The melanocyte to basal keratinocyte ratio varies from 1:10 on truncal skin to 1:3 on facial skin, and the ratio is affected by solar damage/sun exposure as well as anatomic site. Melanocytes produce melanin, which has an ultraviolet light-protective function. Melanin is packaged in melanosomes that are exported via slender, elongated, dendritic processes that extend between keratinocytes.
Langerhans cells are usually histologically invisible and function as antigenpresenting cells. They are suprabasal dendritic cells that, when seen in aggregates,
have a coffee-bean shaped nucleus. Langerhans cells are visible only via special stains or in aggregates in chronic inflammatory disorders and in Langerhans cell histiocytosis. Merkel cells are located along the stratum basale and are also histologically invisible. They have recently been determined to be derived from progenitor keratinocytes and are presumed to serve in tactile perception.
Small, slender, regularly spaced downward extensions of the epidermis (rete) divide the superficial dermis into papillae. The papillary dermis, located immediately beneath the basement membrane, is composed of fine collagen and elastic tissue fibers and contains the capillary loops of the vascular plexus. The reticular dermis, with its haphazardly arranged thick collagen bundles and elastic tissue fibers, is separated from the papillary dermis by the superficial vascular plexus. Elastic fibers, a component of both the papillary and the reticular dermis, are usually visible only with the aid of special stains. The dermis provides structural support and flexibility to the skin.
The dermis also contains adnexal structures, arrector pili muscles, nerves, and blood vessels. Adnexal structures in the skin include hair follicles and the eccrine, apocrine, and sebaceous glands. Eccrine glands develop as downgrowths of the epidermis. They are present as secretory coils in the deep dermis and have a vertically oriented duct that communicates directly through the epidermis by way of a pore called the acrosyringium. Alternatively, apocrine glands develop from the follicular unit and communicate to the surface via a connection through the follicular infundibulum. Like the eccrine gland they have a deep coil and a vertically oriented duct, although the apocrine coil has a larger central space than the eccrine coil and has the classic eosinophilic cytoplasmic apical bleb. Like the apocrine gland, sebaceous glands are outgrowths of the follicular infundibula. They form lobules that connect to the follicle via a small duct and are composed of peripheral basaloid cells and central mature sebocytes with vacuolated cytoplasm.
Hair follicles have varied features depending on the type of hair they produce and, if they are a terminal hair, whether they are actively growing (anagen), resting (telogen) or involuting (catagen). The infundibular portion of the follicle is histologically identical to the epidermis. This portion extends from the epidermal surface to the sebaceous duct. The isthmus has trichilemmal keratinization and extends from the sebaceous duct to the insertion point of the arrector pili muscle (the bulge). The lower segment of the follicle extends from the bulge to the bulb, the classic ball-and-claw that is seen at the base of every hair follicle.
The subcutis is composed of mature adipose tissue separated into lobules by fibrous septae. Fully lipidized adipocytes have a slender, crescentic, barely visible nucleus that has been displaced to the periphery of the cell by the accumulated lipid. The deep vascular plexus separates the subcutis from the reticular dermis.
II. COMMON DESCRIPTIVE TERMS
B. Acanthosis: Thickening of the epidermis (e-Fig. 38.2).
C. Bulla: Fluid containing space in the epidermis, >1cm in size.
D. Dyskeratosis: Abnormal keratinization that results in altered eosinophilic cytoplasm; individual dyskeratotic cells may be referred to as Civatte or colloid bodies (e-Fig. 38.3).
E. Epidermotropism: Migration of malignant cells into the epidermis (e-Fig. 38.4).
F. Exocytosis: Migration of benign, nonepithelial cells into the epidermis, commonly seen in association with spongiosis (e-Fig. 38.5).
G. Hypergranulosis: Thickening (increased number of layers) of the granular layer (e-Fig. 38.6).
H. Hyperkeratosis: Thickening of the stratum corneum.
I. Orthokeratosis: Appropriately mature stratum corneum composed of superficial keratinocytes without nuclei. Seen in a characteristic loose “woven” (basketweave) pattern on nonacral skin, and densely compact on the acral skin of the palms and soles (e-Fig. 38.7).
J. Parakeratosis: Abnormally retained keratinocyte nuclei in the stratum corneum (e-Fig. 38.8).
K. Spongiosis: Fluid/edema creating a space between adjacent cells in the stratum spinosum, which makes the desmosomes appear prominent (e-Fig. 38.9).
L. Vacuolar change: Clearing of basal keratinocyte cytoplasm secondary to inflammation at the epidermal-dermal junction (e-Fig. 38.10).
III. GROSS EXAMINATION AND TISSUE SAMPLING. The skin biopsy/excision is generally received in the laboratory in a fixative such as 10% formalin. If special studies are required, a nonfixative preservative (e.g., Michel’s medium) is required. The gross description should include all pertinent information, including tissue size in centimeters (length × width × thickness), presence or absence of epidermis, color, presence or absence of hair (especially if from the scalp), and alterations to the epidermal surface (including documentation of the dimensions, color, and distance to the nearest margin of discrete lesions). All surfaces, except the epidermis, must be inked before sectioning. Avoiding the use of black ink facilitates interpretation of commonly used special stains, immunostains, natural pigments, and some exogenous pigments. If the clinician has provided orientation for specific margin identification, inking with two or more colors is required.
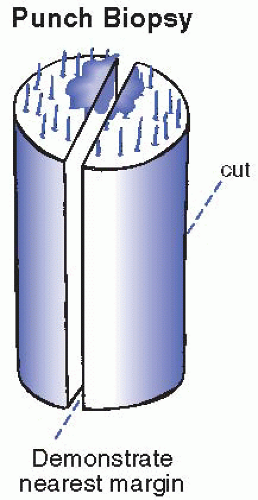
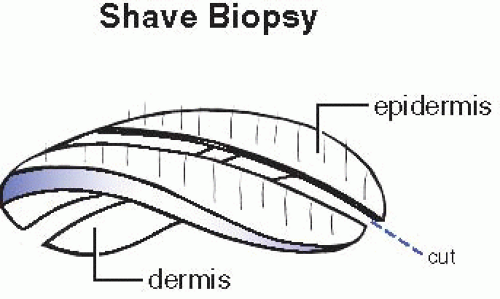
Shave or punch biopsies with a greatest epidermal dimension of <0.3 cm are submitted for processing without sectioning. Specimens with a greatest epidermal measurement of at least 0.4 cm are sectioned vertically through the epidermis resulting in pieces of relatively uniform thickness (˜0.2 to 0.3 cm thick) (Figs. 38.1 and 38.2). As seen in Fig. 38.1, if an epidermal lesion is present, sectioning that will best represent the lesion and its relationship to the nearest margin is optimal. Biopsy tissue is otherwise sectioned along the longest epidermal axis, thus maximizing microscopic visualization (which is particularly important when incisional/wedge biopsies are performed).
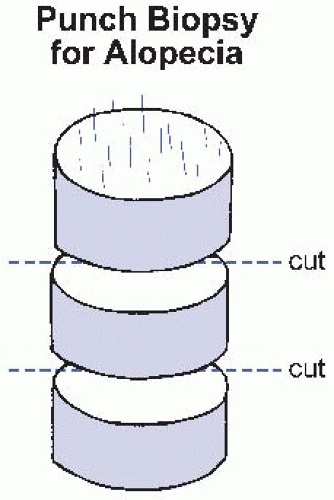
Punch biopsies of the scalp for evaluation of alopecia are sectioned horizontally (every 0.2 to 0.3 cm) to permit evaluation of follicular density and architecture at various tissue levels (Fig. 38.3). One plane of section should separate the tissue at the level of the deep reticular dermis. The two pieces of tissue are placed with each superficial surface down in the tissue cassette and are subsequently embedded with this same orientation. This allows sections that include complete sequential discs that include en face sections of all of the follicles in the specimen. If embedded and sectioned appropriately, the superficial sections will also include a peripheral rim of epidermis and basement membrane zone. The deeper levels will highlight the infundibulum and isthmus; the lower segment will highlight the hair bulb.
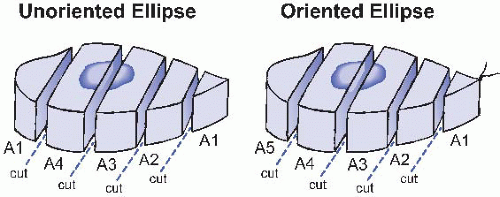
Elliptical biopsies and excisions are approached, in general, in a similar fashion. If an ellipse is oriented by a suture or any other means, inks of different colors are applied to the two long margins to allow specific margin identification under the microscope. Sections of an ellipse should be taken at regular intervals of 2 to 3 mm to allow a reasonable assessment of the true margin. Laboratories vary in their handling of the tip ends of ellipses; the recommended method is illustrated in Figure 38.4. To prevent embedding errors, cassettes should never be overcrowded. Sponges can help prevent distortion of the tissue during processing; for example, submit punch biopsies on one sponge, and lay the pieces of a shave biopsy flat between two sponges. If alternative embedding is required, as for alopecia biopsies, clear instructions to the histology technicians are helpful in ensuring appropriate sections.
Frozen sections may be helpful in evaluating margins of cutaneous carcinomas and may (rarely) be requested if a life-threatening condition (such as toxic epidermal necrolysis [TEN]) requires tissue diagnosis prior to treatment. Frozen sections for diagnosis or margin examination of melanocytic neoplasms are never indicated and may compromise diagnosis on the basis of subsequent permanent sections. The majority of dermatopathology diagnoses are best made on adequately processed, fixed tissue.
IV. INFLAMMATORY DERMATOSES
A. Lichenoid/interface: Characterized by basal keratinocyte damage; may have a band of lymphocytes in the upper dermis (lichenoid) or show only vacuolar alteration of basal keratinocytes and dyskeratosis (interface).
1. Lichen planus
a. Clinical: Multiple, flat topped papules and plaques, pruritic, sometimes featuring superficial white lines (Wickham’s striae), most common in adults, can involve skin, hair, nails, and mucous membranes.
b. Microscopic: Compact hyperkeratosis, acanthosis, band of lymphocytes at the epidermal-dermal junction, dyskeratotic keratinocytes in (Civatte bodies) and under (colloid bodies) the epidermis, rete with a “saw-tooth” pattern, melanophages (e-Fig. 38.11).
2. Lichen planus-like keratosis
a. Clinical: A single red, scaly plaque on sun-damaged skin (arms and chest/shoulders); the clinical impression is frequently that of a basal cell carcinoma or other nonmelanoma skin cancer.
b. Microscopic: Basketweave orthokeratosis with patchy parakeratosis, obscuring band of lymphocytes at the epidermal-dermal junction, flattened/atrophic epidermis sometimes, vacuolar alteration, dyskeratosis, melanophages (e-Fig. 38.12A and B).
3. Erythema multiforme (EM)/Stevens-Johnson syndrome (SJS)/TEN
a. Clinical: EM is a clinical reaction pattern characterized by symmetric, targetoid lesions seen best on the palms and soles; it may involve the mucosa, and it is associated with multiple “triggers” (most commonly HSV). SJS and TEN always have mucosal ulcerations with cutaneous sloughing. SJS involves <10% of body surface area (BSA) whereas TEN >30% BSA.
b. Microscopic: Vacuolar alteration of basal keratinocytes, aggregates of dyskeratotic keratinocytes throughout the epidermis, possible subepidermal bulla with full-thickness epidermal necrosis, lymphocytes in the epidermis and around the superficial vessels (e-Fig. 38.13).
4. Lupus erythematosus
a. Clinical: Lupus can involve the skin only or involve multiple organ systems. Skin findings, which vary with the clinical disease, include a “butterfly”-shaped erythematous rash on the cheeks and nose, scaly erythematous lesions on sun-exposed skin and/or scarring, and alopecic plaques.
b. Microscopic: The spectrum of changes includes epidermal atrophy, follicular plugging, vacuolar alteration of basal keratinocytes, scattered dyskeratotic keratinocytes, thickened basement membrane, superficial and deep perivascular and periadnexal infiltrate of lymphocytes, some extravasated erythrocytes, and interstitial dermal mucin (e-Fig. 38.14). Direct immunofluorescence of a well-established lesion usually shows linear-granular immunoglobulin G (IgG), IgM, and/or complement deposition at the basement membrane.
5. Graft versus host disease (GVHD)
a. Clinical: GVHD may be hyperacute (rarely, <14 days from transplant), acute, or chronic. Acute cutaneous GVHD is often a morbilliform eruption with sudden onset that favors acral sites (palms, soles, head, and neck) and may occur before gastrointestinal or hepatic manifestations. Chronic GVHD may clinically resemble lichen planus (lichenoid GVHD) or morphea (sclerodermoid GVHD).
b. Microscopic: Acute GVHD shows epidermal atrophy with a spectrum of changes at the basement membrane zone (see the section on grading). Because the process is mediated by lymphocytes, lymphocytes in the epidermis and superficial dermis are required for the diagnosis. The histologic changes of acute GVHD are indistinguishable from those seen in response to marrow engraftment (cutaneous eruption of lymphocyte recovery). Grading of acute GVHD:
Grade 0: No histologic alteration by skin biopsy. Grade 1: Vacuolar alteration.
Grade 2: Dyskeratotic keratinocytes in the epidermis and/or adnexal epithelium (e-Fig. 38.15).
Grade 3: Clefting of the epidermis from the dermis.
Grade 4: Complete separation of the epidermis from the dermis.
Grades 1 to 4 require the presence of lymphocytes in the dermis and epidermis (the number of lymphocytes may vary); eosinophils may also be present and do not necessarily support the diagnosis of a reaction to a medication.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree