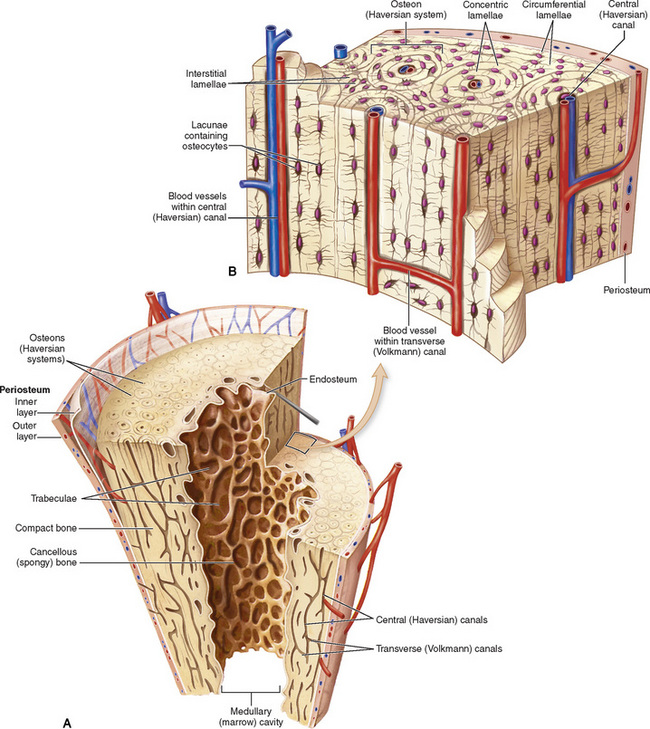
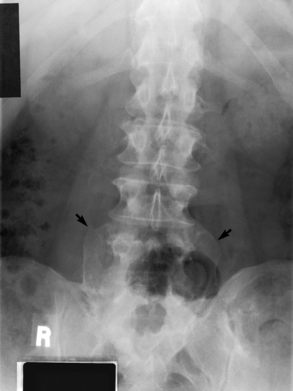
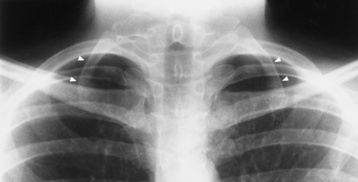
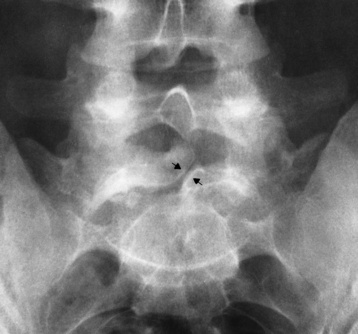
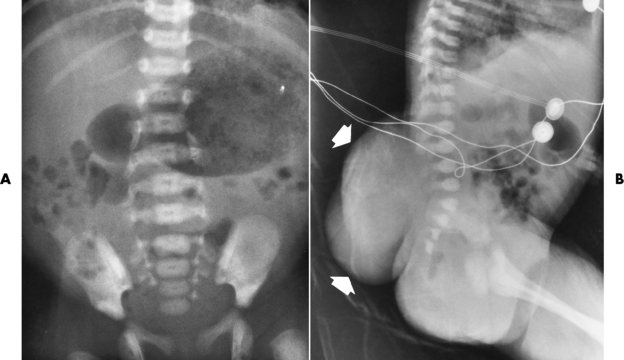
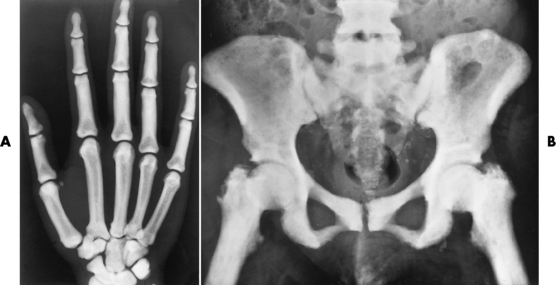
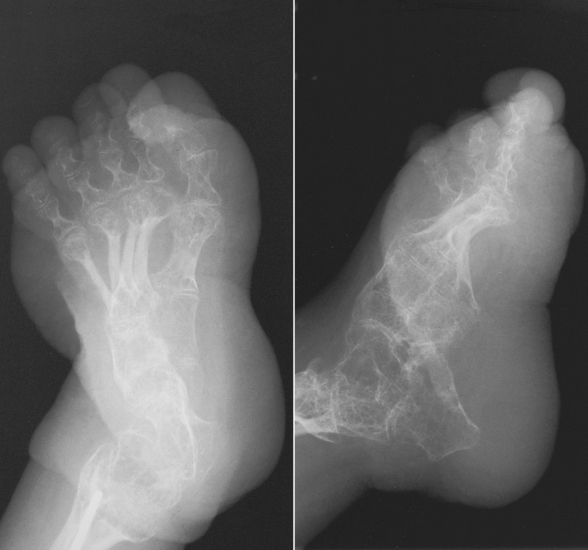
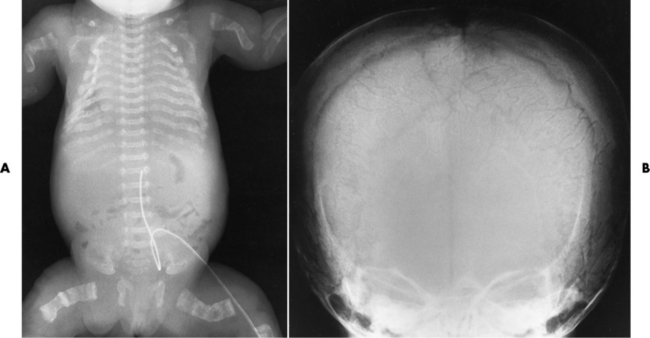
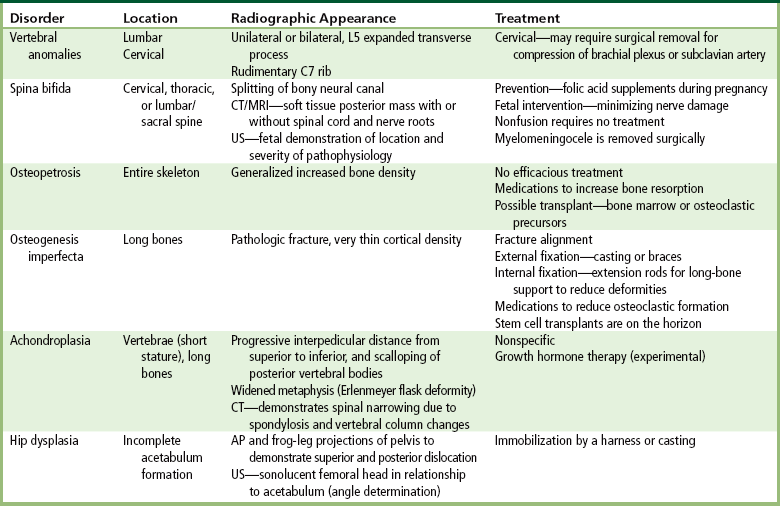
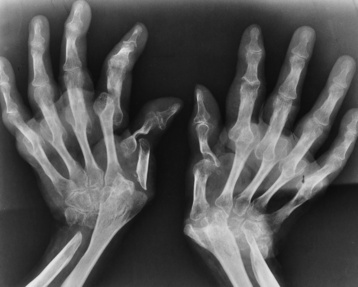
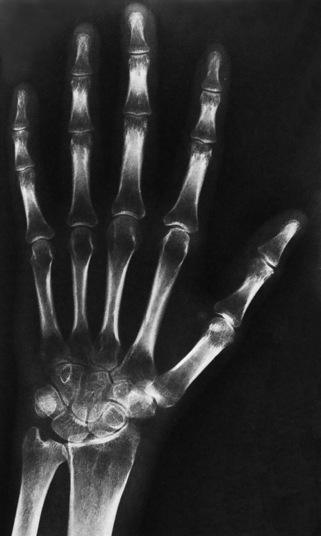
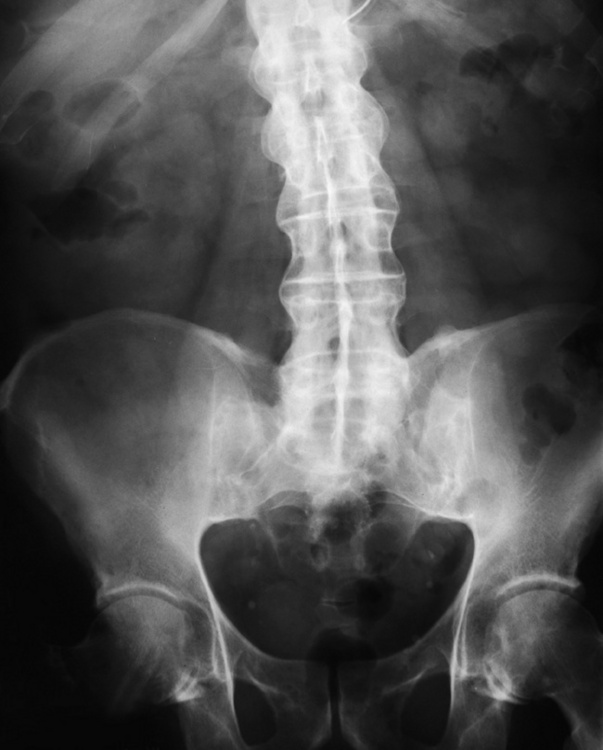
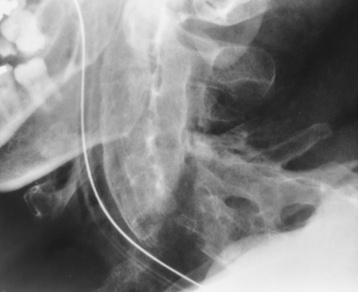
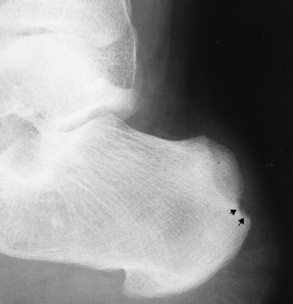
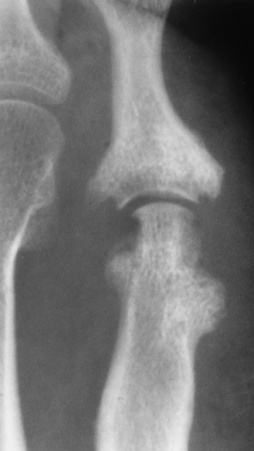
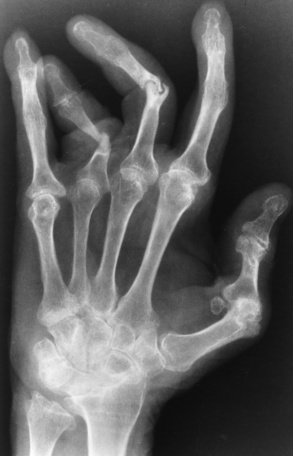
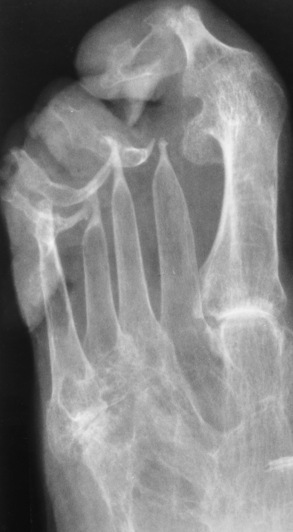
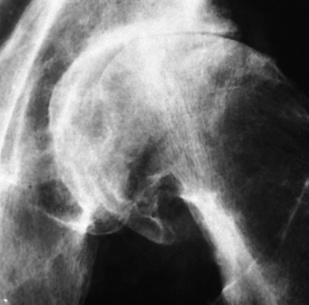
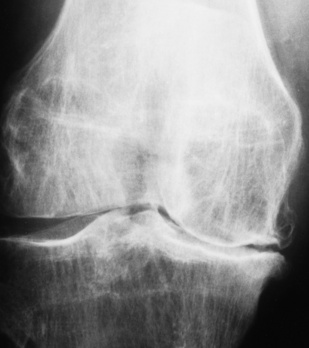
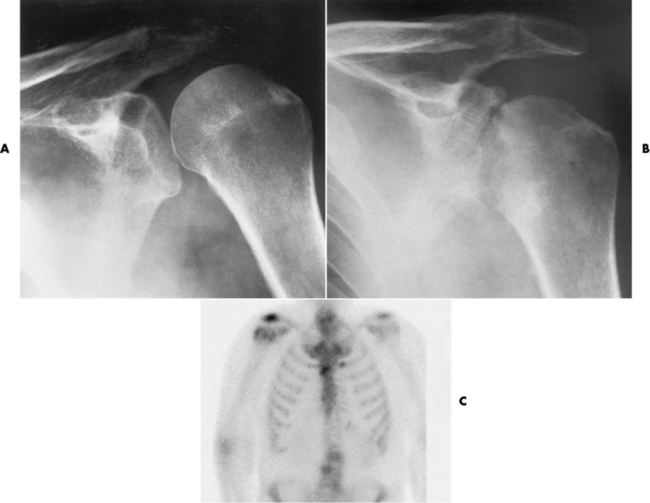
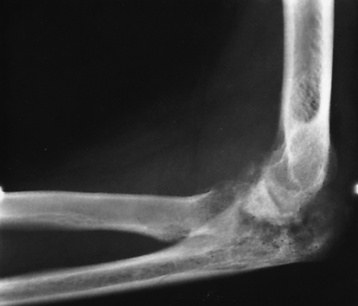
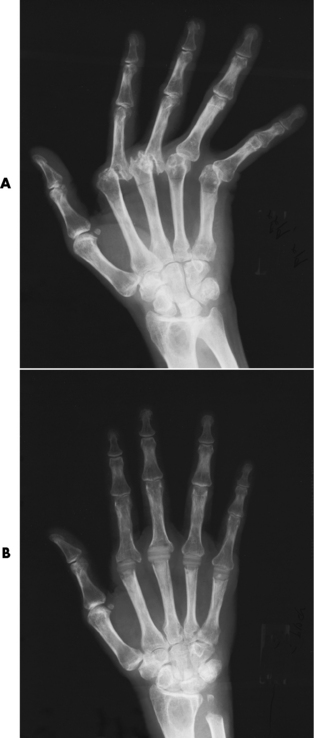
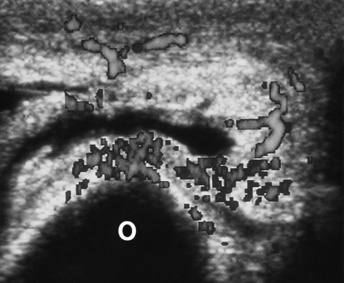
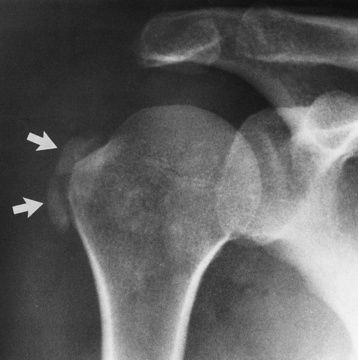
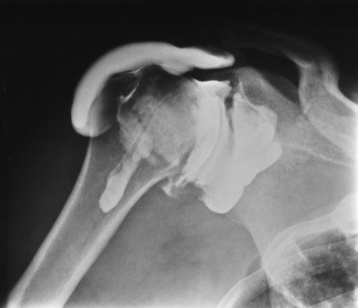
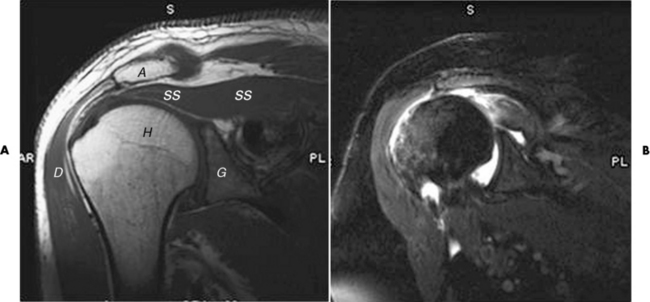
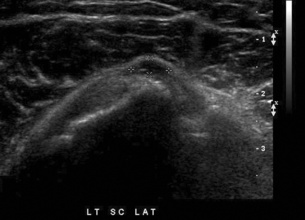
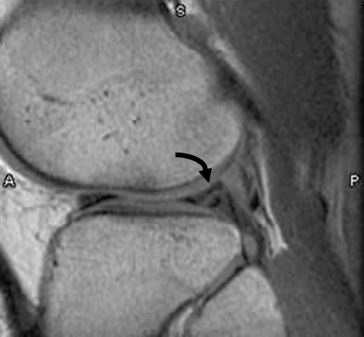
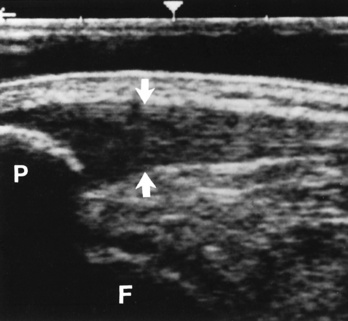
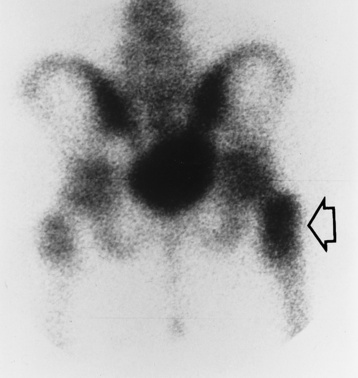
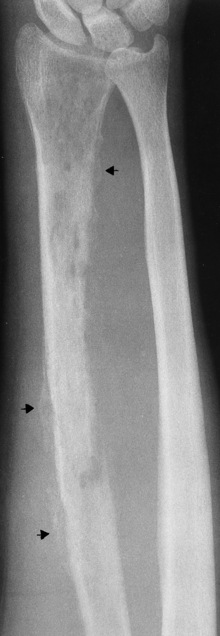
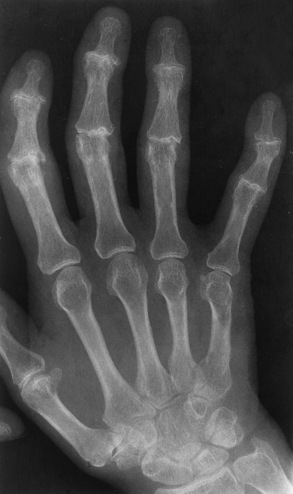
Chapter 4 Physiology of the Skeletal System Congenital/Hereditary Diseases of Bone Inflammatory and Infectious Disorders Rheumatoid Variants: Ankylosing Spondylitis, Reiter’s Syndrome, and Psoriatic Arthritis Osteoarthritis (Degenerative Joint Disease) Tears of the Menisci of the Knee Fractures and Dislocations of the Spine Herniation of Intervertebral Disks After reading this chapter, the reader will be able to: 1 Classify the more common diseases in terms of their attenuation of x-rays 2 Explain the changes in technical factors required for obtaining optimal-quality images in patients’ with various underlying pathologic conditions 3 Define and describe all bold-faced terms in this chapter 4 Describe the physiology of the skeletal system 5 Identify anatomic structures on both diagrams and radiographs of the skeletal system 6 Differentiate the various pathologic conditions affecting the skeletal system and their radiographic manifestations The skeletal system is composed primarily of two highly specialized connective tissues: bone and cartilage. Bone consists of an organic matrix in which inorganic salts (primarily calcium and phosphate) are deposited. A fibrous membrane termed the periosteum covers the outer surfaces of bone, except at joint surfaces, where articular cartilage covers the bone and acts as a protective cushion. The periosteum contains a network of blood vessels from which nutrient arteries penetrate into the underlying bone. The main shaftlike portion is termed the diaphysis, and the ends of the bone are called epiphyses. The hollow, tubelike structure within the diaphysis, known as the medullary cavity, or marrow, is lined by an inner membrane termed the endosteum (Figure 4-1A). There are two major types of bone. The outer layer consists of compact bone, which to the naked eye appears dense and structureless. Under the microscope, the matrix of compact bone consists of complex structural units called “haversian systems.” Cancellous (spongy) bone is composed of a weblike arrangement of marrow-filled spaces separated by thin processes of bone, called trabeculae, that are visible to the naked eye (Figure 4-1B). The relative amount of each type of bone depends on the strength required and thus varies from bone to bone and in different portions of the same bone. For example, the shafts of long bones, such as the femur and tibia, have a thick outer layer of compact bone, whereas the layer of compact bone is relatively thin in irregular bones, such as vertebral bodies and facial bones, and in short bones, such as the carpal and tarsal bones. Most bones form from models composed of hyaline cartilage (enchondral ossification). In a typical long bone, a primary ossification center appears in the center of the cartilage precursor in about the eighth week of intrauterine life, and bone formation extends so that the entire shaft is usually ossified before birth. Just before or after birth, secondary ossification centers appear in the epiphyses, the ends of developing long bones. Until the linear growth of bone is complete, the epiphysis remains separated from the diaphysis by a cartilaginous plate called the epiphyseal cartilage. The epiphyseal cartilage persists until the growth of the bone is complete. At that time, the epiphyseal plate ossifies, and the epiphysis and diaphysis fuse (at various ages depending on the specific bones). Where the diaphysis meets the epiphyseal growth plate is a slight flaring, known as the metaphysis. The increase in length of a developing long bone occurs by growth of the epiphyseal cartilage followed by ossification. Bones grow in diameter by the combined action of two special types of cells called osteoblasts and osteoclasts. Osteoclasts enlarge the diameter of the medullary cavity by removing bone from the diaphysis walls. At the same time, osteoblasts from the periosteum produce new bone around the outer circumference. Osteoblasts and osteoclasts thus continuously resorb old bone and produce new bone. This constant process of remodeling occurs until the bone assumes its adult size and shape. Throughout life, bone formation (ossification) and bone destruction (resorption) continue to occur. They are in balance during the early and middle years of adulthood. After about age 40 years, however, bone loss at the inner or endosteal surface exceeds bone gain at the outer margins. Thus in long bones, the thickness of compact bone in the diaphyses decreases, and the diameter of the medullary cavity increases. The bone eventually resembles a hollow shell and is less able to resist compressive and bending forces. This process may lead to collapse and loss of height of vertebral bodies as well as to fractures of long bones after relatively mild injury. Bones can also develop within a connective tissue membrane (intramembranous ossification). The clavicles and flat bones of the skull have no cartilaginous stage and begin to take shape when groups of primitive cells differentiate into osteoblasts, which secrete matrix material and collagenous fibrils. Deposition of complex calcium salts in the organic bone matrix produces rodlike trabeculae that join in a network of interconnecting spicules to form spongy or cancellous bone. Eventually, plates of compact or dense bone cover the core layer of spongy bone. Flat bones grow in size by the addition of osseous tissue to their outer surfaces (appositional growth). They cannot grow by expansion, as enchondral bone does. Bones perform five basic functions (Box 4-1). A transitional vertebra is one that has characteristics of vertebrae on both sides of a major division of the spine. Transitional vertebrae most frequently occur at the lumbosacral junction and contain expanded transverse processes, which may form actual unilateral or bilateral joints with the sacrum (Figure 4-2). When unilateral, this process often leads to degenerative change involving the opposite hip and the intervertebral disk space above it. At the thoracolumbar junction, the first lumbar vertebra may have rudimentary ribs articulating with the transverse processes. The seventh cervical vertebra may also have a rudimentary rib (Figure 4-3). Spina bifida refers to a posterior defect of the spinal canal, resulting from failure of the posterior elements to fuse properly. A mild, insignificant form is spina bifida occulta, in which there is a splitting of the bony neural canal at the L5 or S1 level (Figure 4-4). Large defects are associated with spinal cord abnormalities and may lead to a variety of muscular abnormalities and lack of bladder or bowel control. In many cases a slight dimpling of the skin or a tuft of hair over the vertebral defect indicates the site of the lesion. Large defects in the lumbar or cervical spine may be accompanied by herniation of the meninges (meningocele), or of the meninges and a portion of the spinal cord or nerve roots (myelomeningocele). A patient with a meningocele may be asymptomatic. Other malformations associated with a meningocele are clubfoot, gait disturbances, and bladder incontinence. The myelomeningocele has associated neurologic deficits at and below the site of protrusion. Almost all patients with myelomeningocele have the Chiari II malformation, with caudal displacement of posterior fossa structures into the cervical canal. Hydrocephalus is a frequent complication. Spina bifida lesions are associated with large bony defects, absence of the laminae, and increased interpedicular distance (Figure 4-5). The herniated spinal contents are seen as a soft tissue mass posterior to the spine. Myelography, computed tomography (CT), or magnetic resonance imaging (MRI) can demonstrate the presence of spinal cord or nerve roots within the herniated sac. Prenatal real-time ultrasound can now demonstrate this serious malformation in utero on sagittal spine fetal images that determine the location and severity of the deformity. Osteopetrosis results in a symmetric, generalized increase in bone density (Figure 4-6). To produce a diagnostic image, the radiographer must increase the exposure factors (milliampere seconds [mAs] and kilovolts peak [kVp]) to compensate for the increase in bone formation (increased attenuation factor). The image may appear blurred because of the structural changes; in some cases, a good image may be difficult to produce. Osteogenesis imperfecta (brittle bones) is an inherited generalized disorder of connective tissue characterized by multiple fractures and an unusual blue color of the normally white sclera of the eye. Due to imperfectly formed or inadequate bone collagen, the adult patient with osteogenesis imperfecta is generally wheelchair bound because the skeletal structure does not support the body weight (Figure 4-7). Patients with this condition suffer repeated fractures caused by the severe osteoporosis and the thin, defective cortices (Figure 4-8A). The fractures often heal with exuberant callus formation (often so extensive as to simulate a malignant tumor), sometimes causing bizarre deformities. Because of the severe cortical bone loss in advanced stages of disease, producing a good radiographic image may require lowering the kilovoltage to compensate for the loss of bone quality. Ossification of the skull progresses slowly, leaving wide sutures and multiple juxtasutural accessory bones within a suture (wormian bones) that produce a mosaic appearance (Figure 4-8B). “Child abuse” may be confused with osteogenesis imperfecta because of the presentation of multiple fractures in different stages of the healing process. The aim of treatment is to reduce fractures by using proper safety measures. Because of defective cortices in severe cases, the long bones become less supportive. Therefore in some instances, extendable rods are surgically placed to provide more support, help prevent new fractures from occurring, and prevent long bone bowing (Figure 4-9). Currently, drugs are prescribed to regulate the osteoclastic formation, thus keeping the bone density more normal. As research continues, stem cell transplants may become a viable cure. Typical radiographic findings include progressive narrowing of the interpedicular distances from above downward, which is the opposite of normal, and scalloping of the posterior margins of the lumbar vertebral bodies (Figure 4-10). The decreased enchondral bone formation may make the long bones appear short and thick with a widened metaphysis (Erlenmeyer flask deformity) (Figure 4-11). CT is beneficial in demonstrating the degree of spinal narrowing caused by spondylosis and the changes in the vertebral column. Anteroposterior (AP) pelvis and bilateral frog-leg (Cleaves) views are required to make a diagnosis (Figure 4-12). In many cases the AP image appears almost normal with only a slightly larger joint space. On the bilateral frog-leg view, the hip is usually dislocated superiorly and posteriorly. Because children with this disorder will undergo a multitude of follow-up images to recheck development, it is very important to shield the gonadal anatomy. Rheumatoid arthritis begins as an inflammation of the synovial membrane (synovitis) that lines the joints. The excessive exudate, a result of the inflammation, causes proliferation of the synovium. The resulting mass of thickened granulation tissue (pannus, meaning “covers like a sheet”) causes erosion of the articular cartilage and underlying bony cortex, fibrous scarring, and even the development of ankylosis (bony fusion across a joint). The erosion occurs because the inflammatory cells produce lytic enzymes. A combination of the fusion of joint surfaces and an inflammatory laxity of ligaments leads to the development of crippling deformities in the end stage of the disease (Figure 4-13). The earliest radiographic evidence of rheumatoid arthritis is fusiform periarticular soft tissue swelling caused by joint effusion and hyperplastic synovial inflammation. Disuse and local hyperemia (increased blood flow) lead to periarticular osteoporosis that initially is confined to the portion of bone adjacent to the joint but may extend to involve the entire bone (Figure 4-14). Extension of the pannus from the synovial reflections onto the bone causes characteristic small foci of destruction at the edges of the joint, where articular cartilage is absent. These typical marginal erosions have poorly defined edges without a sclerotic rim and sometimes may be seen only on oblique or magnification projections. Destruction of articular cartilage causes narrowing of the joint space. The laying down of bony trabeculae across a narrow joint space may completely obliterate the joint cavity and produce solid bony ankylosis, which most frequently involves the bones of the wrist (see Figure 4-13). Ligamentous involvement produces a variety of contractures and subluxations causing the common ulnar deviation of the hands (see Figure 4-13). In the cervical spine, rheumatoid arthritis characteristically produces atlantoaxial subluxation (Figure 4-15), an increased distance between the anterior border of the odontoid and the superior border of the anterior arch of the atlas (normally less than 2.5 mm), which results from weakening of the transverse ligaments from synovial inflammation. The synovial inflammation appears as a soft tissue mass that causes a narrowing of the atlantoaxial articulation on CT images. CT also demonstrates associated erosion of the odontoid (in about two thirds of patients). Radiographic Appearance of Ankylosing Spondylitis Ankylosing spondylitis almost always begins in the sacroiliac joints, causing bilateral and usually symmetric involvement. Blurring of the articular margins and patchy sclerosis generally progress to narrowing of the joint space and may lead to complete fibrous and bony ankylosis (Figure 4-16). The disease typically progresses from the lumbar spine upward. Figure 4-16 Ankylosing spondylitis. Bilateral, symmetric obliteration of the sacroiliac joints and “bamboo spine.” Ossification in the paravertebral tissues and longitudinal spinal ligaments (poker spine) combines with extensive lateral bony bridges (syndesmophytes) between vertebral bodies to produce the characteristic “bamboo spine” of advanced disease (Figure 4-17; see Figure 4-16). Limitation of activity leads to generalized skeletal osteoporosis and a tendency to fracture in response to the stress of minor trauma (Figure 4-18). CT demonstrates both the fusion due to intraarticular and ligamentous ossification and any spinal stenosis caused by the displacement of the fractures. Reiter’s syndrome (reactive arthritis) is characterized by arthritis, urethritis, and conjunctivitis. It primarily affects young adult men and appears to occur after certain types of venereal or gastrointestinal infections. Reiter’s syndrome most frequently involves the sacroiliac joints, heel, and toes (Figure 4-19). Unlike in ankylosing spondylitis, the sacroiliac involvement here is usually bilateral but asymmetric, and Reiter’s syndrome tends to cause only minimal changes in the spine. Although the radiographic changes in peripheral joints often mimic rheumatoid arthritis, Reiter’s syndrome tends to be asymmetric and primarily involves the feet rather than the hands (Figure 4-20). Psoriatic arthritis refers to a rheumatoid arthritis–like destructive process involving peripheral joints that develops in patients with typical skin changes of psoriasis (Figure 4-21). Unlike rheumatoid arthritis, psoriatic arthritis predominantly involves the distal rather than the proximal interphalangeal joints of the hands and feet, produces asymmetric rather than symmetric destruction, and causes little or no periarticular osteoporosis. Characteristic findings include bony ankylosis of the interphalangeal joints of the hands and feet and resorption of the terminal tufts of the distal phalanges (Figure 4-22). Characteristics common to both Reiter’s syndrome and psoriatic arthritis include erosions and hypertrophic changes occurring in the origin and insertion of the tendons and ligaments. In the fingers, degenerative joint disease involves primarily the distal interphalangeal joints (Figure 4-23). Marginal spurs produce well-defined bony protuberances that appear clinically as the palpable and visible knobby thickening of Heberden’s nodes. In the hip, the most prominent finding is asymmetric narrowing of the joint space that involves predominantly the superior and lateral aspects of the joint, where the greatest stress of weight bearing exists (Figure 4-24). Joint space narrowing is also asymmetric in the knee, where it predominantly involves the medial femorotibial compartment (Figure 4-25). Figure 4-23 Osteoarthritis of fingers. Note narrowing of the interphalangeal joints with spurring and erosions. Soft tissue swelling is the first radiographic sign of acute bacterial arthritis. In children, fluid distention of the joint capsule may cause widening of the joint space and actual subluxation, especially about the hip and shoulder. Periarticular edema displaces or obliterates adjacent tissue fat planes (Figure 4-26A). Rapid destruction of articular cartilage causes joint space narrowing early in the course of the disease. The earliest bone changes, which tend to appear 8 to 10 days after the onset of symptoms, are small focal erosions in the articular cortex. Because of the delay in bone changes, detection of the characteristic soft tissue abnormalities is essential for early diagnosis. Severe, untreated infections cause extensive destruction and a loss of the entire cortical outline (see Figure 4-26). With healing, sclerotic bone reaction results in an irregular articular surface. If the articular cartilage has been completely destroyed, bony ankylosis usually follows. Tuberculous arthritis is a chronic, indolent (organic) infection that has an insidious (gradual) onset and a slowly progressive course (Figure 4-27). It usually involves only one joint, and it affects primarily the spine, hips, and knees. Most patients have a focus of tuberculosis elsewhere in the body, most commonly in the lungs. Arthritis therapy should protect affected joints, maintain mobility, and strengthen muscles. For this to occur, lifestyle changes, use of support devices, drugs, and surgery may be necessary. For those with rheumatoid arthritis and osteoarthritis, which make up 90% of all cases diagnosed, rest and exercise are recommended to minimize inflammation and preserve the range of motion. The first-line medications are nonsteroidal antiinflammatory drugs (NSAIDs), which decrease the inflammatory response but do not affect the disease process. Prostaglandin inhibitors, such as aspirin and ibuprofen (salicylates), fall into this category of drugs that reduce the triggering of inflammation. A more aggressive group of drugs, disease-modifying antirheumatic drugs, are used in advanced stages to reduce symptoms. The antimetabolite methotrexate, a cytotoxic drug, tempers cell division in the synovial joint. Invasive surgery involves replacing joints with new artificial joints (Figure 4-28) to increase joint mobility. For infectious arthritis, antibiotics usually eradicate the infection and cure the arthritis. Aspiration may be needed if fluid accumulates in the bursa. Bursitis refers to an inflammation of the bursae, small fluid-filled sacs located near the joints that reduce the friction caused by movement. Repeated physical activity commonly causes bursitis, but trauma, rheumatoid arthritis, gout, or infection also can cause this inflammation. Bursitis or tenosynovitis is usually not visualized on plain radiographs, but disorders of the bursa and synovium can be seen on ultrasound images (Figure 4-29). Plain radiographic images may exclude other disorders that cause similar symptoms. The major radiographic manifestation of bursitis is the deposition of calcification in adjacent tendons, which is a common cause of pain, limitation of motion (frozen joints), and disability about a joint. Calcific tendinitis most commonly involves the shoulder, and calcification may be demonstrated radiographically in about half the patients with persistent pain and disability in the shoulder region (Figure 4-30). However, calcification may also be detected in asymptomatic persons, and severe clinical symptoms may occur without evidence of calcification. Calcific tendinitis appears as amorphous calcium deposits that most frequently occur about the shoulder in the supraspinatus tendon, where they are seen directly above the greater tuberosity of the humerus. The deposits vary greatly in size and shape, from thin curvilinear densities to large calcific masses. The rotator cuff of the shoulder is a musculotendinous structure composed of the teres minor, infraspinatus, supraspinatus, and subscapularis muscles. Rupture of the rotator cuff produces a communication between the shoulder joint and the subacromial bursa that can be demonstrated by arthrography (the injection of contrast material directly into the shoulder joint) (Figure 4-31). Currently, MRI is considered the modality of choice for demonstrating a rotator cuff disorder. The normal rotator cuff appears as a black structure, but tears cause it to have high signal intensity (Figure 4-32). However, ultrasound may be the preferred initial modality to demonstrate the tear of the tendon (Figure 4-33) because of its wider availability and lower cost. MRI demonstrates a tear as a sharply marginated line of high signal intensity (Figure 4-34) that crosses the normally dark, triangular meniscus. In addition, this modality can show the often-associated tears of the anterior and posterior cruciate ligaments and changes in the underlying bone. Ultrasound may demonstrate tenosynovitis of related tendons, which appears as a thickened synovial sheath (Figure 4-35). Because the earliest changes of osteomyelitis are usually not evident on plain radiographic images until about 10 days after the onset of symptoms, radionuclide (technetium Tc-99m) bone scanning is the most valuable imaging modality for the early diagnosis of osteomyelitis. Increased nuclide uptake, reflecting the inflammatory process and increased blood flow, is evident within hours of the onset of symptoms (Figure 4-36). On plain radiographs, the earliest evidence of osteomyelitis in a long bone is a localized, deep soft tissue swelling adjacent to the metaphysis. The inflammation causes displacement or obliteration of the normal fat planes adjacent to and between the deep muscle bundles, unlike in skin infections, in which the initial swelling is superficial. The initial bony change appears as subtle areas of metaphyseal lucency reflecting resorption of necrotic bone. Soon, bone destruction becomes more prominent, producing a ragged, moth-eaten appearance (Figures 4-37 and 4-38). The more virulent the organism, the larger the area of destruction. Subperiosteal spread of inflammation elevates the periosteum and stimulates the deposition of layers of new bone parallel to the shaft. This results in a layered periosteal reaction that is characteristic of benign diseases, especially infection. Eventually, a large amount of new bone surrounds the cortex in a thick, irregular bony sleeve (involucrum). Disruption of cortical blood supply leads to bone necrosis. Segments of avascular dead bone (sequestra) remain as dense as normal bone and are clearly differentiated from the demineralized bone, infected granulation tissue, and pus around them (Figure 4-39). Power Doppler ultrasound demonstrates increased vascularity in the inflammation elevating the periosteum.
Skeletal System
Physiology of the skeletal system
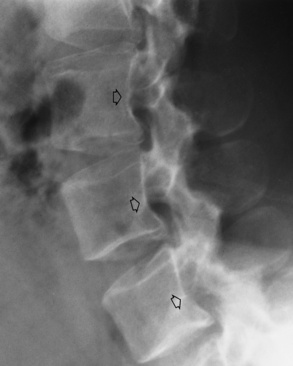
Congenital/hereditary diseases of bone
Radiographic Appearance
Spina Bifida
Radiographic Appearance
Osteopetrosis
Radiographic Appearance
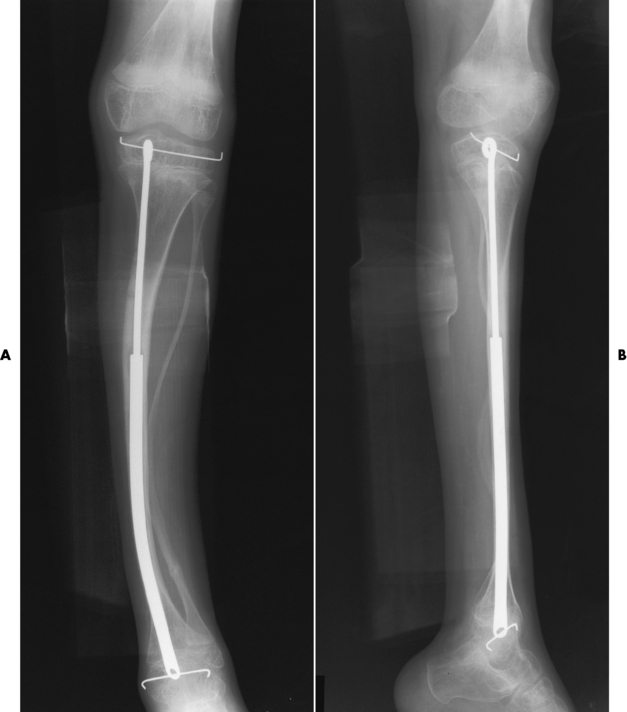
Osteogenesis Imperfecta
Radiographic Appearance
Treatment
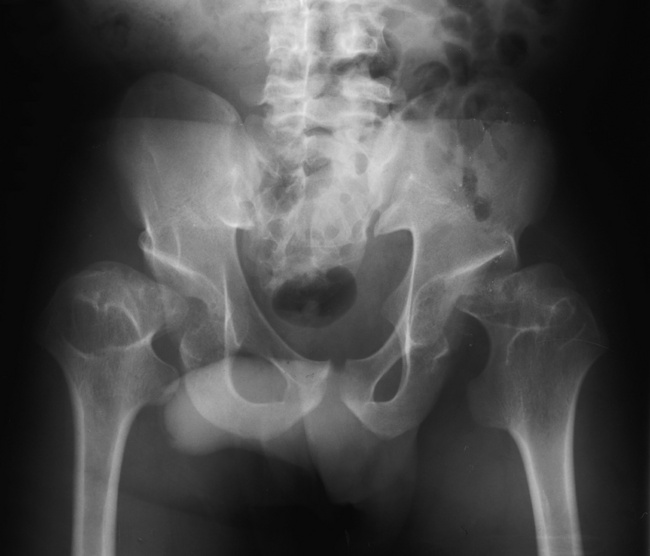
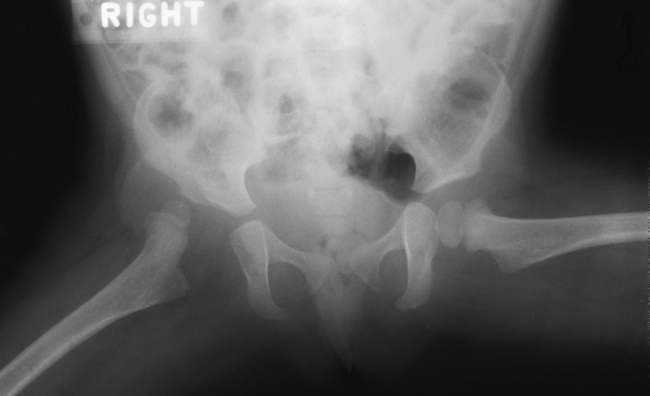
Achondroplasia
Radiographic Appearance
Congenital Hip Dysplasia (Dislocation)
Radiographic Appearance
Inflammatory and infectious disorders
Radiographic Appearance
Rheumatoid Variants: Ankylosing Spondylitis, Reiter’s Syndrome, and Psoriatic Arthritis
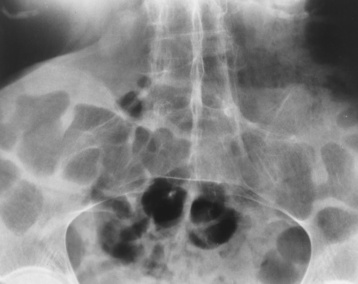
Radiographic Appearance of Reiter’s Syndrome
Radiographic Appearance of Psoriatic Arthritis
Osteoarthritis (Degenerative Joint Disease)
Radiographic Appearance
Infectious Arthritis
Radiographic Appearance
Radiographic Appearance of Tuberculous Arthritis
Treatment of Arthritis
Bursitis
Radiographic Appearance
Rotator Cuff Tears
Tears of the Menisci of the Knee
Bacterial Osteomyelitis
Radiographic Appearance
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Skeletal System