Sexually Transmitted Diseases
KEY CONCEPTS
![]() All recommended treatment regimens for gonorrhea include antibiotic therapy directed against Chlamydia species because of the high prevalence of coexisting infections, unless chlamydia has been ruled out.
All recommended treatment regimens for gonorrhea include antibiotic therapy directed against Chlamydia species because of the high prevalence of coexisting infections, unless chlamydia has been ruled out.
![]() Parenteral penicillin is the treatment of choice for all syphilis infections. For patients who are penicillin allergic, few well-studied alternative agents are available, and all are oral medications that require 2 to 4 weeks of therapy to be effective. Patient compliance and thus efficacy are a concern when alternative regimens must be used.
Parenteral penicillin is the treatment of choice for all syphilis infections. For patients who are penicillin allergic, few well-studied alternative agents are available, and all are oral medications that require 2 to 4 weeks of therapy to be effective. Patient compliance and thus efficacy are a concern when alternative regimens must be used.
![]() Chlamydia genital tract infections represent the most frequently reported communicable disease in the United States. In females, these infections are frequently asymptomatic or minimally symptomatic and, if left untreated, are associated with the development of pelvic inflammatory disease and attendant complications such as ectopic pregnancy and infertility. As a result, all sexually active females aged 20 to 25 years and sexually active women with multiple sexual partners should be screened annually for this infection.
Chlamydia genital tract infections represent the most frequently reported communicable disease in the United States. In females, these infections are frequently asymptomatic or minimally symptomatic and, if left untreated, are associated with the development of pelvic inflammatory disease and attendant complications such as ectopic pregnancy and infertility. As a result, all sexually active females aged 20 to 25 years and sexually active women with multiple sexual partners should be screened annually for this infection.
![]() Oral acyclovir, famciclovir, and valacyclovir are effective in reducing viral shedding, duration of symptoms, and time to healing of first-episode genital herpes infections, with maximal benefits seen when therapy is initiated at the earliest stages of infection. The benefit of these agents for recurrent infections has not been demonstrated. Patient-initiated, single-day antiviral therapy started within 6 to 12 hours of prodromal symptom onset offers an alternative to continuous suppressive therapy of recurrent infection in some individuals.
Oral acyclovir, famciclovir, and valacyclovir are effective in reducing viral shedding, duration of symptoms, and time to healing of first-episode genital herpes infections, with maximal benefits seen when therapy is initiated at the earliest stages of infection. The benefit of these agents for recurrent infections has not been demonstrated. Patient-initiated, single-day antiviral therapy started within 6 to 12 hours of prodromal symptom onset offers an alternative to continuous suppressive therapy of recurrent infection in some individuals.
![]() Metronidazole and tinidazole are the only agents currently approved in the United States to treat trichomoniasis. Although a single 2-g dose of either agent is widely used for compliance and other reasons, the alternative 7-day metronidazole regimen may be a better choice if sexual partners of treated individuals cannot be treated concurrently.
Metronidazole and tinidazole are the only agents currently approved in the United States to treat trichomoniasis. Although a single 2-g dose of either agent is widely used for compliance and other reasons, the alternative 7-day metronidazole regimen may be a better choice if sexual partners of treated individuals cannot be treated concurrently.
The spectrum of sexually transmitted diseases (STDs) has broadened from the classic venereal diseases—gonorrhea, syphilis, chancroid, lymphogranuloma venereum, and granuloma inguinale—to include a variety of pathogens known to be spread by sexual contact (Table 95–1). Because of the large number of infected individuals, the diversity of clinical manifestations, the changing drug-susceptibility patterns of some pathogens, and the high frequency of multiple STDs occurring simultaneously in infected individuals, the diagnosis and management of patients with STDs are much more complex today than they were even a decade ago.1–4
TABLE 95-1 Sexually Transmitted Diseases
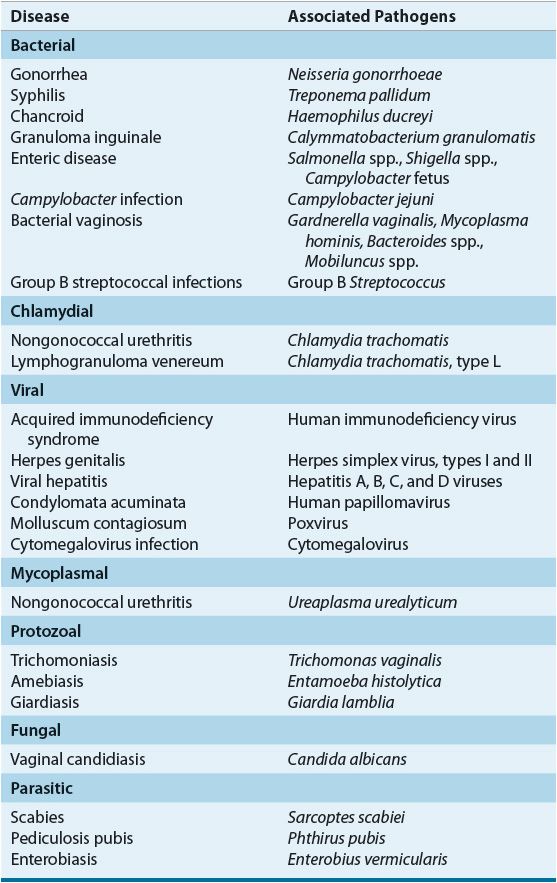
Despite a higher reported incidence of most major STDs in men, the complications of STDs generally are more frequent and severe in women. In particular, serious effects on maternal and infant health during pregnancy are well documented.1,4 Damage to reproductive organs, increased risk of cancer, complications associated with pregnancy, and transmission of disease to the fetus or newborn are associated with several STDs. As a result of the physiologic, psychosocial, and economic consequences of STDs, and because of the increasing prevalence of some viral STDs, such as human immunodeficiency virus (HIV) and genital herpes, for which curative therapy is not available, there is continuing research into STDs and the primary prevention of these diseases.2–5
With the exception of HIV infection, which is reviewed in detail in Chapter 103, the most frequently occurring STDs in the United States are discussed in this chapter. For other less common STDs, only recommended treatment regimens are presented. The most current information on the epidemiology, diagnosis, and treatment of STDs provided by the U.S. Centers for Disease Control and Prevention (CDC) can be obtained at the CDC Website (www.cdc.gov).
Numerous interrelated factors contribute to the epidemic nature of STDs. Sociocultural, demographic, and economic factors, together with patterns of sexual behavior, host susceptibility to infection, changing properties of the causative pathogens, disease transmission by asymptomatic individuals, and environmental factors, are important determinants of the frequency and distribution of STDs in the United States and worldwide.
Age is one of the most important demographic determinants of STD incidence. Two thirds of STD cases each year occur in persons in their teens and twenties, the peak years of sexual activity. With increasing age, the incidence of most STDs decreases exponentially. In sexually active teenagers, STD rates are highest in the youngest, suggesting that physiologic differences may contribute to increased susceptibility.2–5
Age-specific rates of STDs are higher in men than in women; however, reported rates may not represent true gender differences but rather may reflect greater ease of detection in men. In recent years, the ratio of male-to-female cases for most STDs has declined, possibly reflecting improvements in the diagnosis of STDs in asymptomatic women or changes in female sexual behavior following the availability of improved methods of contraception. Although some racial disparity exists for rates of STD infection, it is possible that this is a reflection of socioeconomic differences.1–5
The single greatest risk factor for contracting STDs is the number of sexual partners. As the number of sexual partners increases, the risk of being exposed to someone infected with an STD increases. Sexual preference also plays a major role in the transmission of STDs. For all major STDs, rates are disproportionately greater in men who have sex with men (MSM) than in heterosexuals. In addition, a number of less common STDs, including several caused by enteric protozoans and bacterial pathogens, occur primarily in MSM. The major risk factors for MSM appear to be related to the greater number of sexual partners and the practice of unprotected anal–genital, oral–genital, and oral–anal intercourse. In addition, prostitution and illicit drug use are associated with a higher incidence of most STDs.1–5
Some of the most serious sequelae of STDs are associated with congenital or perinatal infections. Most neonatal infections are acquired at birth, after infant passage through an infected cervix or vagina. Neonatal Chlamydia trachomatis, Neisseria gonorrhoeae, and herpes simplex virus (HSV) infections are associated with this type of spread. For pregnant women with syphilis, infection is usually transmitted transplacentally, producing a congenital infection. Depending on the organism, neonatal infections can manifest in a variety of ways, produce significant morbidity, and in some cases result in infant death.1–4
Other than complete abstinence, the most effective way to prevent STD transmission is by maintaining a mutually monogamous sexual relationship between uninfected partners. Short of this, use of barrier contraceptive methods, such as the male and female condoms, diaphragm, cervical cap, vaginal sponges, and vaginal spermicides alone or in combination, provides varying degrees of protection from a number of STDs. When used correctly and consistently, male latex condoms with or without spermicide are more effective than natural skin condoms in protecting against STD transmission, including HIV, gonorrhea, chlamydia, trichomoniasis, HSV, and human papillomavirus (HPV). When lubrication is desired with latex condoms, water-based products, such as K-Y jelly, are recommended because oil-based agents (e.g., petroleum jelly) can weaken latex condoms and reduce their effectiveness. For latex-allergic individuals, other synthetic condoms (e.g., polyurethane) appear to possess efficacy against STD transmission similar to latex condoms. The female condom is a lubricated polyurethane sheath with a diaphragm-like ring on each end that can be used as a protective device for women with male sexual partners who do not desire to use a condom. Limited data suggest that the female condom blocks penetration of viruses, including HIV; for nonviral STDs, the female condom provides STD protection similar to the male condom.1,3,5,6 At one time, use of nonoxynol-9, a vaginal spermicide with cytolytic activity, was advocated to reduce the transmissibility of several STDs. This was based in large part on in vitro and animal data. However, nonoxynol-9 does not reduce the risk of transmission of common STDs and actually can increase the risk of HIV transmission. Frequent use of nonoxynol-9 damages vaginal, cervical, and rectal epithelium, leading to increased transmissibility of HIV and possibly other STDs. Diaphragms may protect against cervical gonorrheal, chlamydial, and trichomonal infections.1,5–8
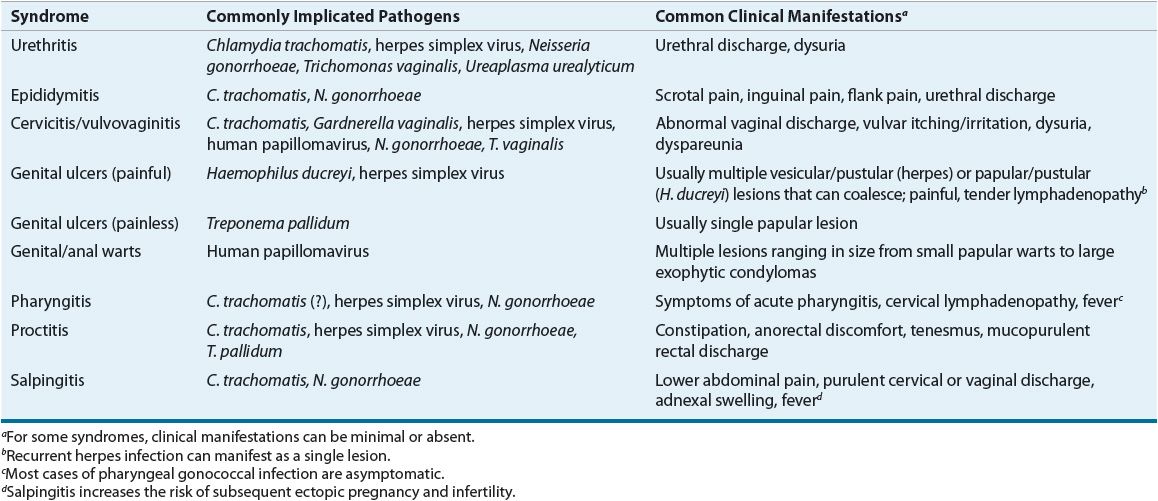
The varied spectrum of clinical syndromes produced by common STDs is determined not only by the etiologic pathogen(s) but also by differences in male and female anatomy and reproductive physiology. For a number of STDs, the signs and symptoms overlap sufficiently to prevent accurate diagnosis without microbiologic confirmation. Frequently, symptoms are minimal or absent despite the presence of infection. Table 95–2 lists common clinical syndromes associated with STDs.1–4
TABLE 95-2 Selected Syndromes Associated with Common Sexually Transmitted Pathogens
GONORRHEA
Epidemiology and Etiology
The gram-negative diplococcus N. gonorrhoeae is the causative organism of gonorrhea. Although the rate of reported cases in the United States has remained relatively stable over the past decade, over 300,000 cases were reported in 2010. However, because of the increasing incidence of resistance to available antibiotics, there is concern that this number may dramatically increase in the future.1,9 Of concern also are the substantial number of infections that remain undiagnosed and unreported.1,9 Humans are the only known natural host of this intracellular parasite. Because of its rapid incubation period and the large number of infected individuals with asymptomatic disease, gonorrhea is difficult to control.1,10–15
Although the risk of a female acquiring a cervical infection after a single episode of vaginal intercourse with an infected male partner is high and increases with multiple exposures, the risk of transmission from an infected female to an uninfected male is not as great following a single act of coitus. No data are available on the risk of transmission after other types of sexual contact.10–14
Pathophysiology
On contact with a mucosal surface lined by columnar, cuboidal, or noncornified squamous epithelial cells, the gonococci attach to cell membranes by means of surface pili and are then pinocytosed. The virulence of the organism is mediated primarily by the presence of pili and other outer membrane proteins. After mucosal damage is established, polymorphonuclear (PMN) leukocytes invade the tissue, submucosal abscesses form, and purulent exudates are secreted.10–15
Clinical Presentation
Individuals infected with gonorrhea can be symptomatic or asymptomatic, have complicated or uncomplicated infections, and have infections involving several anatomic sites. Interestingly, most of the symptomatic patients who are not treated become asymptomatic within 6 months, with only a few becoming asymptomatic carriers of the disease.10–13 The most common clinical features of gonococcal infections are presented in Table 95–3.
TABLE 95-3 Presentation of Gonorrhea Infections
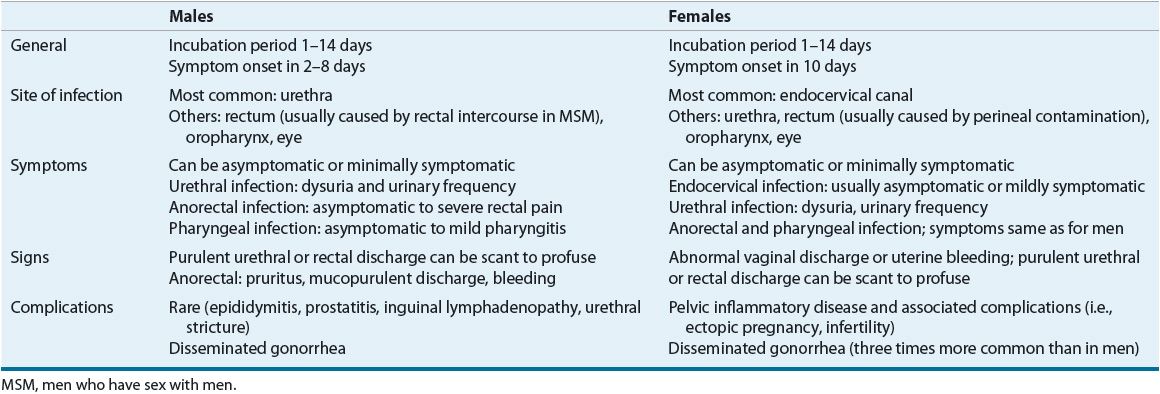
Complications associated with untreated gonorrhea appear more pronounced in women, likely a result of a high percentage who experience signs and symptoms that are nonspecific and minimally symptomatic. As a result, many women do not seek treatment until after the development of serious complications, such as pelvic inflammatory disease (PID). Approximately 15% of women with gonorrhea develop PID. Left untreated, PID can be an indirect cause of infertility and ectopic pregnancies. In 0.5% to 3% of patients with gonorrhea, the gonococci invade the bloodstream and produce disseminated disease. Disseminated gonococcal infection (DGI) is three times more common in women than in men. The usual clinical manifestations of DGI are tender necrotic skin lesions, tenosynovitis, and monoarticular arthritis.1,10–14
Diagnosis
Diagnosis of gonococcal infections can be made by gram-stained smears, culture, or methods based on the detection of cellular components of the gonococcus (e.g., enzymes, antigens, DNA, or lipopolysaccharide [LPS]) in clinical specimens. Various stains have been used to identify gonococci microscopically, with the Gram stain the most widely used in clinical practice. Gram-stained smears are positive for gonococci when gram-negative diplococci of typical kidney bean morphology are identified within PMN leukocytes.1,10–14 In the presence of equivocal smears (extracellular gonococcal forms that can be nonpathogenic, commensal Neisseria, or gram-negative diplococci of atypical morphology), culture is mandatory. In urethral smears from men with symptomatic urethritis, the smear is highly sensitive and specific, and is considered diagnostic for infection. Because of their low sensitivity, gram-stained smears are not recommended in the diagnosis of endocervical, rectal, cutaneous, and asymptomatic male urethral infections. Because of the presence of nonpathogenic Neisseria in the pharynx, the Gram stain is not useful in the diagnosis of pharyngeal infection.1,10,12–14
Although no longer considered the most sensitive of diagnostic tests for gonorrhea, culture is considered the test of choice because of its high specificity in medicolegal situations (e.g., suspected abuse, rape); in diagnosing anorectal, pharyngeal, and conjunctival infections; and in screening populations with a low prevalence. Anatomic sites to be cultured depend on the individual’s sexual preferences and body areas exposed. In women, because the urethra and other sites are rarely the sole locus of infection, cervical cultures produce the highest yield and frequently are performed in conjunction with rectal cultures. Urethral cultures are recommended in women who have had hysterectomies and heterosexual men.1,10–14
Because technical constraints and cost preclude the use of culture techniques in many office settings and clinics, alternative methods of diagnosis have been developed, including enzyme immunoassay (EIA), DNA probe techniques, and nucleic acid amplification techniques (NAATs). With the exception of Gram stain for symptomatic gonococcal urethritis, these tests offer increased sensitivity and/or specificity over both Gram stain and culture.10,13,14,16 Additionally, many of these tests can provide a more rapid means of diagnosis than culture. Of particular clinical importance is the high sensitivity of NAATs for detecting N. gonorrhoeae using noninvasive specimens (e.g., self-collected urine specimens, vaginal swabs). As a result, NAAT is considered the standard of care for the diagnosis of gonorrhea. This technology is also being used to concurrently test for C. trachomatis using a single specimen. However, a major drawback of NAATs is their inability to provide resistance data on isolated gonococcal strains. In cases of documented treatment failure, antimicrobial susceptibility testing is recommended.1,14–16
TREATMENT
![]() In 2010 the CDC issued an update to their recommended treatment regimens for gonorrhea. This update eliminated oral cephalosporins from the recommended treatment regimens for gonorrhea, leaving parenteral ceftriaxone as the only recommended agent for treating gonorrhea14 (Table 95–4). The ceftriaxone-based regimens are the only regimens that have well-documented efficacy in the treatment of urethral, cervical, rectal, and pharyngeal infections. Coexisting chlamydial infection, which is documented in up to 50% of women and 20% of men with gonorrhea, constitutes the major cause of postgonococcal urethritis, cervicitis, and salpingitis in patients treated for gonorrhea for whom concurrent chlamydial infection has not been ruled out.1,14 As a result, concomitant treatment with azithromycin or doxycycline is recommended in all patients treated for gonorrhea. If a cefixime-based regimen is used to treat gonorrhea, it is recommended that patients return in 1 week for a test of cure. While azithromycin (2 g) as a single dose appears highly effective in eradicating both gonorrhea and chlamydia, it is not recommended as a preferred alternative to ceftriaxone because of concerns regarding the development of resistance. In cephalosporin-allergic individuals, azithromycin (2 g) is currently the only alternative available to treat gonorrhea.1,14,17,18
In 2010 the CDC issued an update to their recommended treatment regimens for gonorrhea. This update eliminated oral cephalosporins from the recommended treatment regimens for gonorrhea, leaving parenteral ceftriaxone as the only recommended agent for treating gonorrhea14 (Table 95–4). The ceftriaxone-based regimens are the only regimens that have well-documented efficacy in the treatment of urethral, cervical, rectal, and pharyngeal infections. Coexisting chlamydial infection, which is documented in up to 50% of women and 20% of men with gonorrhea, constitutes the major cause of postgonococcal urethritis, cervicitis, and salpingitis in patients treated for gonorrhea for whom concurrent chlamydial infection has not been ruled out.1,14 As a result, concomitant treatment with azithromycin or doxycycline is recommended in all patients treated for gonorrhea. If a cefixime-based regimen is used to treat gonorrhea, it is recommended that patients return in 1 week for a test of cure. While azithromycin (2 g) as a single dose appears highly effective in eradicating both gonorrhea and chlamydia, it is not recommended as a preferred alternative to ceftriaxone because of concerns regarding the development of resistance. In cephalosporin-allergic individuals, azithromycin (2 g) is currently the only alternative available to treat gonorrhea.1,14,17,18
TABLE 95-4 Treatment of Gonorrhea
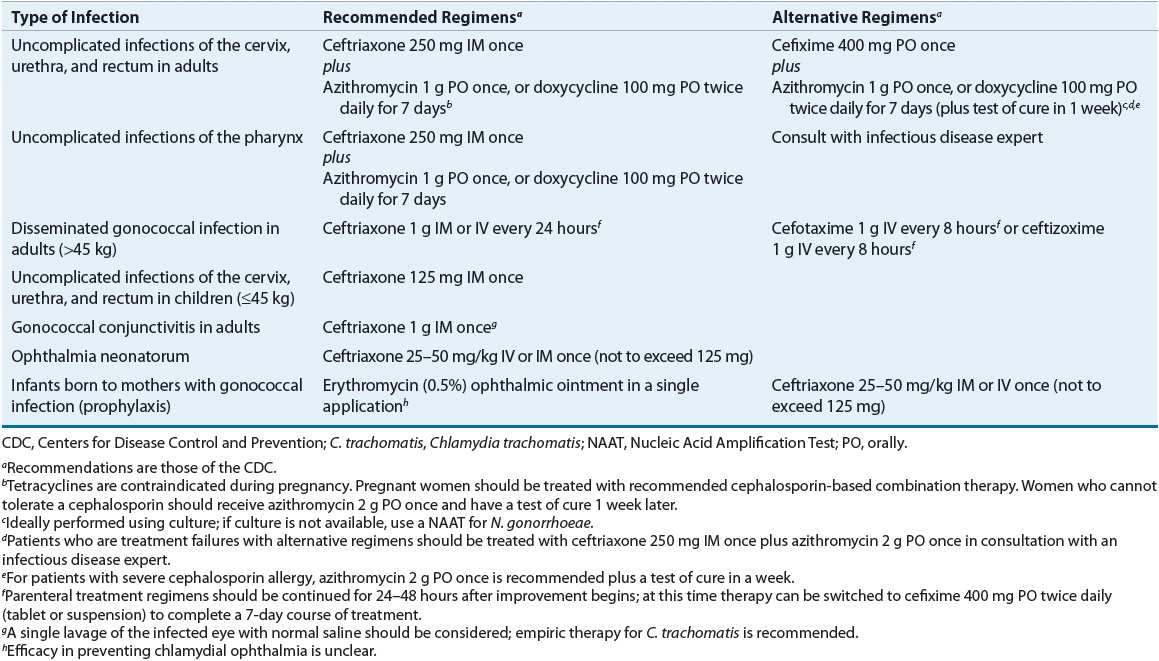
Although oral therapy with cefixime may offer a more patient acceptable alternative to intramuscular ceftriaxone, the declining susceptibility of gonorrheal isolates in the United States to cefixime has resulted in its move from a recommended regimen of choice to an alternative regimen. Additionally, only ceftriaxone is effective in treating pharyngeal gonorrhea and eradicating both gonorrhea and incubating syphilis in a patient coinfected with both organisms. The latter is particularly beneficial in areas with a high rate of syphilis.1,10,12–15
Pregnant women infected with N. gonorrhoeae should be treated with ceftriaxone. For presumed or diagnosed concurrent C. trachomatis infection, azithromycin or amoxicillin is the preferred treatment.1,10,11
Ceftriaxone is the recommended therapy for DGI, gonococcal meningitis, endocarditis, and any type of gonococcal infection in children. In cases of DGI, patients should be hospitalized and treated with ceftriaxone or one of the alternative parenteral cephalosporin antibiotics (see Table 95–4). Although marked improvement is usually noted within 48 hours of initiating therapy, treatment should be continued for at least 7 days.1,13,14 Gonococcal ophthalmia is highly contagious in adults and neonates and requires ceftriaxone therapy. Single-dose therapy is adequate for gonococcal conjunctivitis, although some physicians recommend continuing therapy until cultures are negative at 48 to 72 hours. Topical antibiotics are not sufficiently effective when used alone for ocular infections and are not necessary with appropriate systemic therapy. Infants with any evidence of ocular infection should be evaluated for signs of DGI.1,10,13–14,19
Clinical Controversy…
Treatment of gonorrhea during pregnancy is essential to prevent ophthalmia neonatorum. Gonococcal infection in newborns results primarily from passage through an infected birth canal, but it also can be transmitted in utero. Ophthalmia neonatorum is the most common ophthalmic infection in newborns (1.6% to 12%), although membranes of the vagina, pharynx, or rectum also can become colonized. Conjunctival involvement usually develops within 7 days of delivery and is characterized by intense, bilateral conjunctival inflammation with chemosis. If not treated promptly, corneal ulceration and blindness can develop. Because the law in most states requires neonatal prophylaxis with topical ocular antimicrobials, gonococcal ophthalmia neonatorum is rare in the United States. The CDC recommends that erythromycin (0.5%) ophthalmic ointment be instilled in each conjunctival sac immediately postpartum.1,10–14,19
Evaluation of Therapeutic Outcomes
In the past, persistence of gonorrhea symptoms a short time following treatment with a recommended regimen against gonorrhea usually indicated reinfection rather than treatment failure and, as such, reflected the need for improved patient education and sex partner referral. However, with antimicrobial resistance increasingly being reported in recent years, reinfection can no longer be assumed as the cause. As a result, the CDC recommends that all apparent treatment failures be assessed using culture and sensitivity testing. Persistence of symptoms also can be due to other infectious causes, such as C. trachomatis.1,10–15 While the CDC does not recommend routine follow-up of patients treated with a recommended regimen, it is recommend that any patient treated with an alternative regimen be tested for cure 1 week following treatment.
SYPHILIS
Epidemiology and Etiology
During the 2000s, the number of reported cases of primary and secondary syphilis in the United States has remained relatively constant at around 14,000.9 Of these newly diagnosed cases, two-thirds are reported in MSM. In addition to being highly contagious, syphilis is of major concern because, if left untreated, it can progress to a chronic systemic disease that can be fatal or seriously disabling.20–29 Syphilis usually is acquired by sexual contact with infected mucous membranes or cutaneous lesions, although on rare occasions it can be acquired by nonsexual personal contact, accidental inoculation, or blood transfusion. The causative organism of syphilis is Treponema pallidum, a spirochete. The risk of acquiring syphilis from an infected individual after a single sexual encounter is approximately 50% to 60%. After sexual contact, the organism penetrates the intact mucous membrane or a break in the cornified epithelium, and spirochetemia occurs.21,24,25–29
There is strong evidence of an association between syphilis and HIV infection. Syphilis, similar to other sexually transmitted genital ulcer diseases, can increase the risk of acquiring HIV in exposed individuals. In addition, immunologic defects in HIV-infected individuals can produce an atypical serologic response to syphilis. In particular, the possibility of delayed seroreactivity, markedly elevated serologic titers, and increased false-positive results could complicate the diagnosis, as well as assessment of treatment efficacy, in HIV-positive individuals infected with syphilis. Furthermore, anecdotal evidence suggests that compromised immune function can result in an accelerated progression of syphilis, particularly to neurosyphilis, requiring more aggressive antibiotic therapy in comparison with an immunocompetent host. As a result of this association, the CDC recommends that all patients diagnosed with syphilis be tested for HIV infection.1,20,22–24,26,27
Clinical Presentation
The clinical presentation of syphilis is varied with progression through multiple stages possible in untreated or inadequately treated patients (Table 95–5).
TABLE 95-5 Presentation of Syphilis Infections
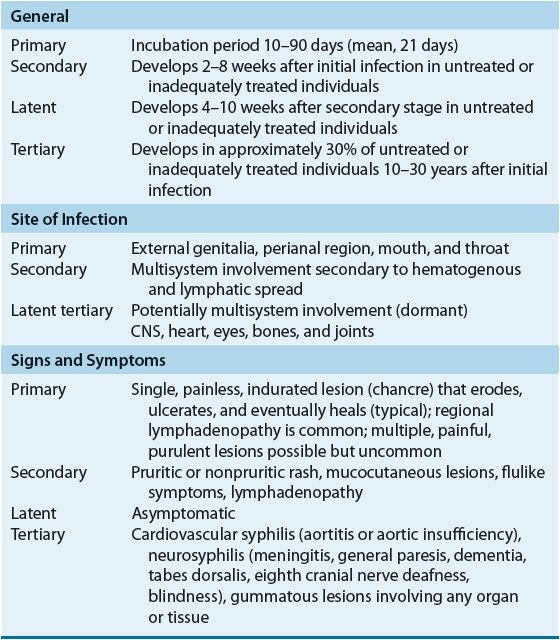
Primary Syphilis
The primary stage, characterized by the appearance of a chancre on cutaneous or mucocutaneous tissue exposed to the organism, is highly infectious. Even without treatment, chancres persist only for 1 to 8 weeks before healing spontaneously. Because syphilitic chancres can be confused with other infectious etiologies, appropriate diagnostic testing is important.20–24,26,28
Secondary Syphilis
The secondary stage of syphilis is characterized by a variety of mucocutaneous eruptions resulting from widespread hematogenous and lymphatic spread of T. pallidum. Skin lesions can be either generalized or localized to a small portion of the body and, with the exception of follicular lesions, are nonpruritic. Generalized lymphadenopathy also is seen in the majority of patients, as are nonspecific symptoms such as mild and transitory malaise, fever, pharyngitis, headache, anorexia, and arthralgia. If untreated, secondary syphilis disappears in 4 to 10 weeks; however, lesions can recur at any time within 4 years.20–28
Latent Syphilis
By definition, persons with a positive serologic test for syphilis but with no other evidence of disease have latent syphilis. Latent syphilis is further divided into early and late latency. During early latency, the patient is considered potentially infectious because of the 25% risk of spontaneous mucocutaneous relapse. The U.S. Public Health Service defines early latency as 1 year from the onset of infection, although other investigators propose a longer interval, such as 2 to 4 years. With the exception of pregnancy in which the mother can pass the disease to the fetus, late latency is considered noninfectious, although the patient remains a host.1,20–28
Most untreated patients with late latent syphilis have no further sequelae; however, approximately 25% to 30% progress either to neurosyphilis or to late syphilis with clinical manifestations other than neurosyphilis. Treatment of all patients with latent syphilis is essential because there is no way to predict which patients will have progression of their disease.20–28
Tertiary Syphilis and Neurosyphilis
If left untreated, syphilis can slowly produce an inflammatory reaction in virtually any organ in the body. Manifestations of this disease progression were referred to previously as tertiary syphilis. These clinical manifestations now are differentiated into two subgroups based on the presence or absence of CNS involvement: neurosyphilis or tertiary syphilis (i.e., gumma and cardiovascular syphilis).1,20–28
Currently, the term neurosyphilis encompasses any patient with cerebrospinal fluid (CSF) abnormalities consistent with CNS infection. Approximately 40% of patients with primary or secondary syphilis exhibit such abnormalities, although most remain asymptomatic. Persistence of CSF abnormalities into late latency is associated with a greater risk of progression to symptomatic neurosyphilis. Although data are conflicting, some investigators suggest that HIV-infected patients are at greater risk of developing symptomatic neurosyphilis than patients with intact immune systems.1,20–28
Rarely seen, the most common manifestations of disease progression from late latency are benign gumma formation and cardiovascular syphilis. The gumma, a nonspecific granulomatous lesion, is the classic lesion of late syphilis and develops in 50% of patients with disease progression. These chronic, destructive lesions characteristically infiltrate the skin, bone, soft tissue, and liver but can be found in any organ or tissue. Gummas of critical organs, such as the heart or brain, can be fatal.1,20–24,27
Congenital Syphilis
In pregnant women with syphilis, T. pallidum can cross the placenta at any time during pregnancy. The risk of fetal infection is greatest in pregnant women with primary and secondary syphilis and declines in pregnant women with late disease. Transmission of syphilis during pregnancy occurs primarily transplacentally and can result in fetal death, prematurity, or congenital syphilis. Symptoms can be seen during the first months of life (early congenital syphilis) or later in childhood or adolescence (late congenital syphilis). Manifestations of early congenital syphilis resemble those of secondary syphilis, whereas those of late congenital syphilis correspond to the tertiary stage in adults.20,22–24
Diagnosis
Because T. pallidum is difficult to culture in vitro, diagnosis is based primarily on microscopic examination of serous material from a suspected syphilitic lesion or on results from serologic testing. In primary syphilis, diagnosis is established by the presence of T. pallidum on dark-field microscopic examination of material from cutaneous lesions and enlarged lymph nodes in patients with secondary syphilis. In incubating syphilis, confirmation frequently is by dark-field microscopic examination because serologic tests can be unreactive early in the disease. Another method of direct microscopic examination, the direct fluorescent-antibody (test) for T. pallidum (DFA-TP), which uses monoclonal or polyclonal antibodies specific for T. pallidum, has greater specificity and sensitivity than dark-field examination, and does not require the immediate examination of fresh specimens.24–29
Serologic tests are the mainstay in the diagnosis of syphilis and traditionally are categorized as nontreponemal or treponemal. Common nontreponemal tests include the Venereal Disease Research Laboratory (VDRL) slide test, rapid plasma reagin (RPR) card test, unheated serum reagin (USR) test, and the toluidine red unheated serum test (TRUST). Nontreponemal tests, which are inexpensive and easily performed, rely on the detection of treponemal antibodies directed against an alcoholic solution of cardiolipin, lecithin, and cholesterol contained in these tests. A positive nontreponemal test can indicate the presence of any stage of syphilis or congenital syphilis, although incubating syphilis and very early primary syphilis produce a negative reaction; however, because they are nonspecific tests, false-positive reactions occur, making them inappropriate to confirm the diagnosis alone. Transiently false-positive results can be seen in patients with acute febrile illnesses, after immunizations, and during pregnancy. Chronic false-positive results are commonly associated with heroin addiction, aging, chronic infections, autoimmune diseases, and malignant disease. In some cases, false-positive reactions are familial and are related to abnormal serum globulin levels.23–29
Nontreponemal tests are used primarily as screening tests; however, because T. pallidum antibody titers also can be quantitated by testing serial dilutions of the patient’s serum for reactivity, they are useful in following the progression of the disease, recovery after therapy, and possible reinfection. Because antibody titers vary to some extent between tests, it is important that sequential serologic testing be performed using the same method each time. In patients treated successfully for primary and secondary syphilis, nontreponemal tests almost always will return to seronegativity. If these tests are going to return to negative in patients with early latent syphilis, they will do so within the first 4 years after adequate therapy; patients with disease of longer duration usually remain seropositive for life. In addition to their use in serologic testing, nontreponemal tests often are used on CSF to diagnose neurosyphilis.23–29
In some patients with secondary syphilis, a prozone phenomenon occurs that produces a negative VDRL test despite the presence of high reaginic antibody titers. This is corrected by diluting the patient’s serum prior to testing.26,27 For HIV-positive individuals with syphilis, the reactivity of nontreponemal tests can vary depending on the stage of the HIV infection. In the early stages, reaginic titers higher than in non–HIV-infected patients have been seen, resulting in the prozone phenomenon. During the later stages of HIV infection, however, when immune function deteriorates to a greater extent, serologic responses can be reduced or delayed. As a result, the diagnosis of syphilis in HIV-infected individuals can be more difficult.1,24–29
In diagnosing all stages of syphilis, treponemal tests are more sensitive than nontreponemal tests. Because these tests are technically more demanding and are more expensive, they are used primarily as confirmatory rather than as screening tests. For many years, the fluorescent treponemal antibody absorption (FTA-ABS) test was the most frequently used treponemal test. The FTA-ABS test uses the T. pallidum antigen to detect specific antibodies to treponemal organisms. However, the FTA-ABS test has largely been replaced by card assays such as the T. pallidum hemagglutination assay (TPHA), the microhemagglutination assay for antibodies to T. pallidum (MHA-TP), and the T. pallidum particle agglutination assay (TPPA) which can be automated and are less expensive to perform. Despite adequate antibiotic therapy for any stage of syphilis, the antibody tests usually remain reactive for life and therefore are not useful in assessing serologic response to therapy, relapse, or reinfection.1,24–29
Several EIAs for T. pallidum have become available and are gaining wide use as confirmatory tests. Polymerase chain reaction (PCR)-based tests also are being investigated, particularly in situations in which serologic testing has poor sensitivity and specificity (e.g., congenital syphilis, early primary syphilis, and neurosyphilis). Additionally, multiplex PCR tests that can identify the presence of T. pallidum, herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2), and Haemophilus ducreyi from genital ulcer specimens are under study. The CDC recommends that all patients diagnosed with syphilis be tested for HIV infection.1,22–24,29
TREATMENT
Table 95–6 presents the CDC’s treatment recommendations.1 Parenteral penicillin G is the treatment of choice for all stages of syphilis. Because T. pallidum multiplies slowly, single doses of short- or intermediate-acting penicillins do not provide the prolonged, low-level exposure to penicillin required for eradication of the treponeme. As a result, benzathine penicillin G is the only penicillin effective for single-dose therapy.1,22–28
TABLE 95-6 Drug Therapy and Follow-up of Syphilis
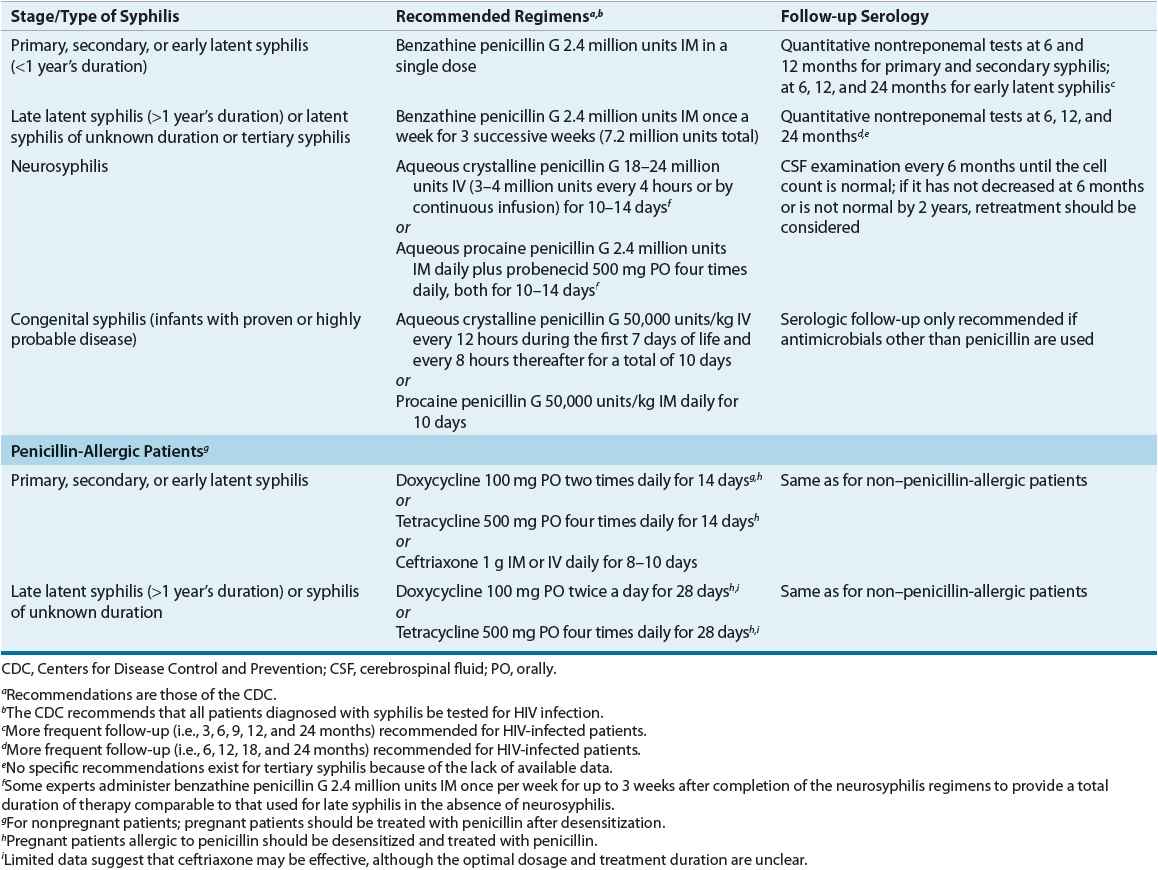
The recommended treatment for syphilis of less than 1 year’s duration is benzathine penicillin G 2.4 million units as a single dose. Although the relapse rate for this regimen is less than 3%, some investigators advocate that 2.4 million units be administered once a week for two consecutive weeks. In patients with syphilis of longer than 1 year’s duration and normal CSF examination, benzathine penicillin G is administered weekly for three successive doses. Although not specifically recommended by the CDC, this three-dose regimen is used by some experts to treat HIV-infected patients with syphilis of less than 1 year’s duration based on data suggesting a greater risk of treatment failure with single-dose therapy.1,24–28
Patients with abnormal CSF findings should be treated as having neurosyphilis. Preferred regimens for neurosyphilis provide treatment over 10 to 14 days with parenteral penicillin G administered every 4 hours. Benzathine penicillin G alone in standard weekly doses and procaine penicillin G in doses under 2.4 million units do not consistently provide treponemicidal levels in the CSF and have resulted in treatment failures. Because T. pallidum resistance to penicillin has not emerged, the primary need for alternative drugs in treating syphilis is for penicillin-allergic patients.1,24–28
![]() Alternative regimens recommended for penicillin-allergic patients are doxycycline 100 mg orally twice daily or tetracycline 500 mg orally four times daily for 2 to 4 weeks depending on the duration of syphilis infection. These regimens should be used only in cases of documented penicillin allergy, and given concerns regarding patient compliance with these regimens, follow-up serologic testing is of particular importance.1,24–28
Alternative regimens recommended for penicillin-allergic patients are doxycycline 100 mg orally twice daily or tetracycline 500 mg orally four times daily for 2 to 4 weeks depending on the duration of syphilis infection. These regimens should be used only in cases of documented penicillin allergy, and given concerns regarding patient compliance with these regimens, follow-up serologic testing is of particular importance.1,24–28
Other antibiotics used successfully in treating syphilis include various β-lactam antibiotics; however, none offers significant advantages over benzathine penicillin G. Even though ceftriaxone is considered effective in eradicating incubating syphilis when given as a single 125-mg dose, higher doses and more frequent administration (e.g., 1,000 mg daily for 8 to 10 days) appear necessary for more advanced syphilis, and treatment failures are reported in HIV-infected patients. Although azithromycin 2 g as a single dose produces good results in patients with early syphilis, treatment failures and resistance to azithromycin are reported.1,21,24–28



