Chapter 9. Sepsis, shock and trauma
Sepsis and infection 242
Fluid balance, shock and anaphylaxis 249
Major trauma 254
Fractures and minor trauma 259
Acute leg pain 270
Wounds and burns 272
SEPSIS AND INFECTION
Definitions
This classification is important because severe sepsis and septic shock are life-threatening emergencies that can progress rapidly to multi-organ failure and death.
Clinical assessment and immediate management
Start by assessing ABC. Thereafter, take a brief history of recent events, past medical and drug history (including allergies) from the patient and/or relatives. Look for clinical evidence of a source of infection and any haemodynamic disturbance or hypoperfusion. Patients with sepsis tend to be vasodilated (warm, bounding peripheral and forearm pulses ± hypotension).
All patients with hypotension and/or evidence of organ hypoperfusion must be treated aggressively with immediate oxygen therapy (see ‘Oxygen delivery’, p. 66) and early, aggressive intravenous fluid resuscitation (see p. 251). In all patients:
• look for obvious clues to the source of the infection: this will help target subsequent investigations and guide empirical antimicrobial prescribing
• if the patient is unwell, administer appropriate broad-spectrum intravenous antibiotics as soon as possible (take blood cultures just before the first dose, if not already done); see ‘Antibiotics’ below for advice on the choice of empirical agent
• speed is important: call for senior help early including HDU or ICU input in patients with severe sepsis, septic shock or dangerous infections such as meningitis
• consider inotropic support in patients with septic shock (see p. 252).
Secondary survey
A more detailed assessment, focusing on possible sources of infection, can be undertaken once you are confident that the patient is stable. Aspects of the initial history and examination may need to be expanded. Friends and relatives may give you important additional information. Important pointers in this evaluation are summarized in Table 9.1, overleaf.
| Presentation | Consider | Comments |
|---|---|---|
| Sore throat ± cervical lymphadenopathy | Bacterial or viral pharyngitis; glandular fever | Consider streptococcal bacteraemia if very unwell |
| Acute watery diarrhoea | Viral infection or salmonella, campylobacter, C. difficile | Ask about food and travel history, contact with young children or animals, recent antibiotics, achlorhydria or PPI therapy |
| Acute bloody diarrhoea | Campylobacter, E. coli 0157, shigella or acute diverticulitis | |
| Abnormal LFTs ± rigors and RUQ tenderness | Ascending cholangitis; malaria if travel history; hepatitis | Ask about travel; viral hepatitis unlikely if febrile |
| Headache, otherwise reasonably well | Common with viral infections and many others, e.g. sinusitis | Common symptom but consider possibility of neurosepsis |
| Headache with photophobia ± neck stiffness, vomiting and confusion | Meningitis | Seek senior help early. Rash suggests meningococcal septicaemia. Meningitis and encephalitis are difficult to distinguish – empirical treatment for both often needed. Follow local protocol |
| Headaches with fits and confusion rather than meningism | Viral encephalitis; severe bacterial meningitis; cerebral malaria if travel history | |
| Loin pain ± rigors | Pyelonephritis | Renal ultrasound ± KUB |
| Erythema, heat, swelling of skin and soft tissues | Cellulitis | Can be difficult to distinguish from DVT |
| As above, but with marked pain ± septic shock | Necrotizing fasciitis | Rare but serious; urgent surgical review required |
| New murmur, cutaneous signs, e.g. splinter haemorrhages, risk factors present | Endocarditis | Ensure 3 sets of blood cultures are obtained plus echo, ESR and CRP |
| Acute mono-arthritis or back pain | Septic arthritis | Look for signs of cord compression if spine involved |
| Rash | Meningococcal infection, streptococcal infection, viral exanthems | Remember non-infectious causes, e.g. drug rash |
Investigations
The following investigations should be performed in all patients, but should not delay resuscitation and immediate management, if the patient is unwell:
• FBC, Coag, U&E, glucose, LFT, CRP, lactate
• ABG if SaO 2 <92%, if shocked or evidence of hypoperfusion
• blood cultures, urinalysis and MSU, together with other site-specific samples, e.g. throat swab, sputum and stool
• CXR and ECG
• in returning travellers, thick and thin blood films for malaria or an antigen blood test (for P. falciparum) should be sent; the test available will depend on local laboratory policy; other conditions to consider and relevant investigations are summarized in Table 9.2 on p. 245
| Presentation | Consider | Comments |
|---|---|---|
| Headache ± rigors and CNS symptoms | Malaria | Send blood for thick and thin films ± antigen |
| Headache, abdominal symptoms; occasionally a faint rash (‘rose spots’) | Typhoid/ paratyphoid | Difficult to distinguish from malaria; send blood cultures |
| Cough and dyspnoea | Legionnaires’ disease, viral RTI, avian influenza | Avian ’flu must be considered if returning from an endemic area |
| Haemorrhagic features | Viral haemorrhagic fever | Very rare; major clinical and infection control challenge |
• other tests may be appropriate in certain clinical settings, e.g. echocardiography in suspected endocarditis.
Antimicrobial therapy
Most hospitals have a site-specific prescribing policy for empirical antimicrobial therapy that incorporates national guidelines and local drug resistance data. An example is provided in Table 9.3 (see p. 245), but you should refer to local advice, where available.
| aFor many patients on IV therapy, a switch to oral administration after 24–48 h is appropriate. | |||
| bMany hospitals now use a once-daily gentamicin regimen. | |||
| cThere is an increasing trend to use alternatives to cephalosporins. | |||
| dOmitted if the patient is not immunocompromised and is <55 years of age. | |||
| Clinical presentation | Treatment | Dose | Days of therapy |
|---|---|---|---|
| Mild to moderate infection (all doses are for oral therapy) | |||
| Cellulitis | Flucloxacillin + penicillin V | 1–2 g 6-hourly 500 mg 6-hourly | 7–14 |
| Cystitis | Co-amoxiclav | 375 mg 8-hourly | 3 |
| Exacerbation of COPD | Amoxicillin or clarithromycin | 500 mg–1 g 8-hourly 500 mg 12-hourly | 5–7 |
| CAP | Amoxicillin + clarithromycin | 500 mg–1 g 8-hourly 500 mg 12-hourly | 7 |
| Severe infection (all doses are for IV therapya) | |||
| Cellulitis | Flucloxacillin + benzylpenicillin | 1–2 g 6-hourly 1.2–2.4 g 6-hourly | 10–14 |
| Pyelonephritis | Ciprofloxacin ± gentamicin (if severe) | 500 mg 12-hourly Local guidelinesb | 7–14 |
| Intra-abdominal sepsis | Amoxicillin + metronidazole + gentamicin | 1 g 8-hourly 500 mg 8-hourly Local guidelinesb | 7–14 |
| Meningitis | Ceftriaxonec+ amoxicillind± aciclovir (in encephalitis) | 2 g 12-hourly 2 g 6-hourly 10 mg/kg TDS | 7–14 |
| Sepsis, uncertain source | Amoxicillin + metronidazole + gentamicin | 1 g 8-hourly 500 mg 8-hourly Local guidelinesb | 7–14 |
| CAP | Augmentin + clarithromycin ± flucloxacillin | 1.2 g 8-hourly 500 mg 12-hourly 1–2 g 6-hourly | 7–14 |
| Postoperative or aspiration pneumonia | Ceftriaxonec+ metronidazole | 2 g once daily 500 mg 8-hourly | 7–10 |
Always ask about drug allergies and only use IV treatment if the patient has specific indications for this, e.g. inability to swallow or severity criteria. If IV therapy is commenced, aim to switch to oral therapy within 24–48 h where possible. Prolonged IV antibiotic therapy will be required in some situations, e.g. endocarditis, meningitis. Empirical therapy may require modification once culture and sensitivity results are obtained. Do not hesitate to seek senior advice from microbiology or infectious diseases departments.
Infection in the immunocompromised
Immunocompromised patients are susceptible to infection with organisms that would not normally cause disease in healthy individuals. Such ‘opportunistic’ infection can occur because of dysfunction or failure of the host’s immune system, or reflect a breach in the body’s normal physical barriers to infection. The identification of immunodeficiency, of what ever kind, allows appropriate investigations to be undertaken and adequate broad-spectrum antimicrobial treatment to be prescribed, pending culture results.
HIV-infected individuals
HIV infection causes a progressive defect of cell-medicated immunity and opportunistic infection may develop. This has become less common since the introduction of highly active antiretroviral therapy (HAART). However, HIV infection should always be considered in patients with risk factors, particularly those with unusual infections (Table 9.4, p. 246). Infection in HIV patients is a complex area, so seek senior advice early.
| System | Condition(s) |
|---|---|
| Respiratory | Atypical pneumonia (consider Pneumocystis), TB |
| GI | Unexplained weight loss, chronic diarrhoea, oesophageal candidiasis |
| Haematological | Lymphadenopathy, lymphoma, thrombocytopenia |
| Dermatological | Kaposi’s sarcoma, seborrhoeic dermatitis, severe molluscum contagiosum |
| Oral | Candidiasis, oral hairy leukoplakia |
| Others | Acute HIV seroconversion, unusual infections, tumours or neurological |
Acute primary HIV infection
Secondary infection
The incidence of secondary infections correlates closely with the patient’s CD4 count. Patients with early disease and relatively normal counts usually present with infections due to ‘common’ organisms or those found in patients with non-HIV immunocompromise, e.g. pneumococcal infection, TB, syphilis (and other STIs), VZV (shingles), recurrent HSV.
Patients with more advanced disease and lower CD4 counts are susceptible to infection with a wider spectrum of low-virulence opportunistic organisms:
• <500 cells/mm 3: oral candidiasis
• <200 cells/mm 3: PCP (see below), invasive candidiasis, cerebral toxoplasmosis
• <100 cells/mm 3: chronic diarrhoea (cryptosporidiosis), fungal meningitis (cryptococcal)
• <50 cells/mm 3: CMV retinitis, M. avium intracellulare (MAI).
Pneumocystis pneumonia
Pneumocystis jirovecii pneumonia (previously Pneumocystis carinii pneumonia, hence the acronym PCP) usually presents sub-acutely with a dry cough, dyspnoea, fever and bilateral, often subtle, CXR changes. Diagnosis requires a high index of suspicion, confirmed by PCR ± immunofluorescence performed on induced sputum or BAL fluid (see also ‘Breathlessness’, p. 157).
Neutropenic sepsis
Patients undergoing chemotherapy frequently develop drug-induced neutropenia. The risk of infection is increased when the neutrophil count falls below 1.0 × 10 9/L. A variety of infections may occur, including unusual and drug-resistant bacteria, fungi (e.g. candida, aspergillus) and viruses (e.g. VZV, CMV).
Neutropenic patients may become very unwell if treatment is delayed, so early empirical antimicrobial treatment should be started if there are features of sepsis (see above) or if the temperature rises above 38.5 °C once or 38 °C twice in 2 h. Figure 9.1 shows an example of a typical management protocol. Neutropenia without sepsis is managed differently and may not require either antibiotics or admission. Prophylactic antibiotics (e.g. ciprofloxacin) may be used along with further FBC monitoring.
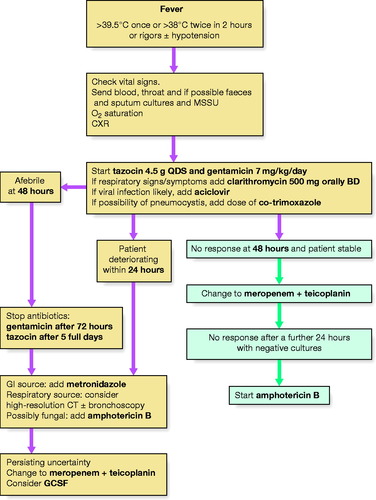 |
| Figure 9.1 Management protocol for neutropenic sepsis. |
Previous splenectomy
Following splenectomy, patients are at an increased risk of invasive bloodstream infections, particularly from S. pneumoniae and other encapsulated organisms such as N. meningitidis and H. influenza. Vaccination is usually offered, e.g. Pneumovac®, and many patients are prescribed low-dose penicillin prophylaxis. They are also at increased risk of severe malaria.
Intravascular devices
Intravascular devices, e.g. peripheral and central lines, constitute a breach of the normal host defences and predispose the patient to skin infection and bacteraemia. Insertion sites should be looked after carefully and inspected regularly. If infection develops, consider S. aureus (including MRSA) and coagulase-negative staphylococcal infection as potential causes. Empirical use of a glycopeptide antibiotic, such as vancomycin, may be indicated for suspected infection if the patient is unwell (refer to local protocol). If an infection develops when a line is already in situ, it may have to be removed.
Pyrexia
The commonest cause of pyrexia is infection, although there are numerous other possible explanations, including drug reactions, malignancy and connective tissue disorders. Conversely, some patients with an infection may not develop a fever, particularly the elderly or immunosuppressed. Indeed, hypothermia is a well-recognized manifestation of severe sepsis.
Pyrexia of unknown origin
Pyrexia of unknown origin (PUO) is defined as a persistent fever, of at least 2 weeks, which remains undiagnosed despite appropriate initial investigations. Potential causes are given in Table 9.5.
| Infectious | Non-infectious |
|---|---|
| TB (pulmonary or non-pulmonary) | Drug reactions |
| Bacterial endocarditis | Malignancy: lymphoma, leukaemia, solid organ (e.g. renal, GI) |
| Intra-abdominal abscesses | Thyrotoxicosis and other endocrine diseases |
| Bone and joint sepsis | Connective tissue disorders, e.g. RA, SLE, Still’s disease |
| Infected implanted medical device | Still’s disease |
| Syphilis | Thromboembolic disease |
| Lyme disease | Alcoholic liver disease |
| Brucellosis | Inflammatory bowel disease |
| Certain viral infections – HIV; CMV | Granulomatous disorders, e.g. sarcoid |
| Fungal infections | Hypothalamic dysfunction |
| Imported infections – malaria; amoebic liver abscess | Factitious fever |
PUO can pose a significant diagnostic challenge. Repeat the history and examination carefully since new findings may emerge. Further investigations should be guided, where possible, by the clinically suspected source, but they are likely to include:
• repeat blood cultures
• samples for AAFB and mycobacterial culture, including sputum, urine and any biopsy material
• serology: chlamydia, mycoplasma, CMV, HIV ± others, e.g. Q-fever, bartonella, brucella
• ANA, RF, ANCA screen
• echocardiogram (transoesophageal if available)
• imaging (US, CT or MRI) ± organ biopsy
• others, where indicated, e.g. lumbar puncture; liver biopsy; bone marrow biopsy; bronchoscopy and lavage; upper and lower GI endoscopy; labelled WBC scan.
Treatment should ideally be guided by the results of these investigations, but empirical antimicrobial therapy may need to be commenced if the patient is deteriorating and/or endocarditis is suspected.
FLUID BALANCE, SHOCK AND ANAPHYLAXIS
Fluid balance
Fluid compartments
Total body fluid is approximately 60% of body weight, meaning that an average 75 kg male contains 45 L of water. This water is contained within distinct fluid compartments within the body. Intracellular fluid (ICF) accounts for two-thirds of body water (30 L). Extracellular fluid (ECF) accounts for one-third of body water (15 L) and is composed of:
• interstitial fluid (ISF) comprises three-quarters of the ECF; surrounds cells and does not circulate
• intravascular fluid comprises one-quarter of the ECF; circulates as the extracellular component of blood
• transcellular fluid accounts for fluid outside normal compartments, e.g. CSF, mucus.
Factors that influence the movement of water between these fluid compartments include pressure, tonicity and the permeability of the barriers in between. The most important site of fluid exchange between fluid compartments is the vascular capillary bed, a schematic of which is presented in Figure 9.2. The structures and physiological factors involved at this level influence fluid balance management in both healthy individuals and those with disease:
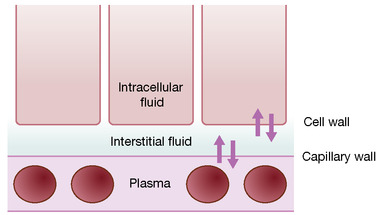 |
| Figure 9.2 The capillary bed. |
Capillary wall
The capillary wall acts as a filter through which water and solutes pass freely; larger molecules including plasma proteins and lipids are unable to pass through. Flow across the capillary wall is determined by the balance between hydrostatic pressure (forcing fluid out of intravascular space; determined blood pressure) and oncotic pressure (sucking fluid back in; generated by plasma proteins). In healthy individuals, the net effect is a small flow of fluid into the interstitial space, producing lymph. However, this balance can be altered in disease causing capillaries to leak protein, e.g. sepsis, resulting in a net loss to the interstitial space and oedema.
Cell wall
Although water moves freely across the cell wall, the movement of solutes is selective. Sodium, the main extracellular cation, is pumped out of cells in exchange for potassium (potassium and magnesium are the main intracellular cations).
Clinical relevance
A basic understanding of the fluid compartments of the body and the way in which fluid is likely to ‘shift’ is important when managing fluid balance. For example, an IV infusion of any fluid will initially increase the intravascular ECF, but the composition of the fluid will dictate its ultimate distribution:
• dextrose is quickly metabolized to water, passing freely throughout the total body water
• normal saline is isotonic with ECF and will increase the volume of interstitial fluid and plasma
• colloid solutions will be less able to diffuse across the capillary membrane and will stay in the intravascular space longer.
Normal maintenance requirements
Normal water requirements are estimated at 20–40 mL/kg per day. In healthy individuals this is achieved by drinking (approx. 1200 mL), eating (1000 mL) and water oxidation (300 mL). Fluid losses are comprised of urine (1500 mL) and insensible losses from skin and lungs (850 mL). The GI tract produces between 6–8 L of fluid/day, but most of it is reabsorbed with only 150 mL lost in faeces.
Fluid resuscitation
Intravenous fluid prescription should be regarded like any other type of prescribing and, where possible, patients should be encouraged to drink. Maintenance requirements and replacement fluids should be considered separately. Consider the following:
• urine output and daily weights are the best monitors of fluid balance
• for every 1 °C rise in temperature give an extra 1 L/day
• 1 L normal saline and 2 L 5% dextrose is a common maintenance regime
• avoid excessive saline infusion as this can cause hyperchloraemic acidosis
• GI secretions are electrolyte rich, so replace with saline like for like
• after surgery K + may rise due to cell damage, so check before supplementing.
See also the general guidance on prescribing fluids, ‘Prescribing blood and fluids’, p. 122.
Shock
Shock is defined as inadequate organ perfusion and tissue oxygenation. While profound shock is easy to recognize, earlier signs can be subtle.
Early signs
• tachycardia: an attempt to preserve cardiac output, reduced in the elderly or those who are β-blocked
• cool or pale peripheries: vasoconstriction reduces blood flow to non-essential organs; in distributive forms of shock (see overleaf) the peripheries may be warm due to vasodilatation
• narrow pulse pressure: diastolic pressure rises to maintain preload and, therefore, cardiac output
• reduced urine output: renal blood flow is reduced and ADH secretion is increased.
Late signs
• reduced consciousness: autoregulation of cerebral blood flow is unable to compensate adequately
• tachypnoea: may indicate metabolic acidosis or ARDS
• negligible urine output.
Mechanisms and causes
Shock can be classified into hypovolaemic, cardiogenic and distributive forms depending on the underlying mechanism.
Hypovolaemic shock
In hypovolaemic shock, there is insufficient circulating blood volume. This may be due to haemorrhage, dehydration, burns or fluid sequestration, e.g. acute pancreatitis.
Cardiogenic shock
This results from primary cardiac pump failure, which may be due to acute myocardial infarction, cardiomyopathy or dysrhythmia.
Distributive shock
The primary problem is diminished systemic vascular resistance (SVR), which may occur for several reasons:
• septic shock: vasodilatation in response to any severe infection (most commonly, Gram-negative endotoxin-secreting bacteria)
• anaphylactic shock: due to vasodilatation as a result of histamine release in response to an allergen, e.g. bee sting
• neurogenic shock: due to loss of vascular autonomic stimulation following spinal cord or brain injury.
Treatment
The general treatment of shock must be aggressive and begin early. Remember the ABCDE approach (see ‘Assessment of the acutely ill patient’, p. 8). Establish a patent airway and ensure adequate oxygenation (>92%): this helps to reverse anaerobic metabolism and metabolic acidosis. Ensure adequate IV access is available (at least two green cannulae) and start IV fluids; see below.
Treat the underlying cause: consider broad-spectrum antibiotics if there is evidence of sepsis (fever, rash, signs of peripheral vasodilatation). If the patient is bleeding, transfuse O-negative blood initially, pending cross-match, and consider definitive intervention to stop bleeding, e.g. laparotomy or urgent endoscopy. Cardiogenic shock and pulmonary oedema should be managed as described in ‘Breathlessness’, p. 146.
Fluids
Fluid resuscitation increases intravascular volume, venous return and cardiac output, but can be detrimental in patients with co-existing cardiac dysfunction or primary cardiogenic shock. Therefore, it is essential to assess intravascular volume state (mucous membranes, skin turgor, JVP, lung bases, oedema) accurately, early and regularly. Facilities should be available for invasive monitoring if bedside assessment of clinical parameters is not felt to be sufficient.
Fluid challenge
Where the patient appears hypovolaemic, give a fluid challenge:
• in adults, run in 250 mL of fluid as rapidly as possible (20 mL/kg in children)
• the speed and volume of the fluid are more important than its composition
• be more cautious in patients with co-existing cardiac disease, in whom central venous access and CVP monitoring should be considered
• it is essential to assess the effect of the challenge as soon it is complete; look at HR, BP, urine output and other measures of tissue perfusion.
Inotropes and vasopressors
Patients who remain hypotensive despite adequate filling should be considered for inotropic or vasopressor support. Therefore, by definition, all should have invasive monitoring of central venous pressure, ideally in a high dependency or intensive care environment. Inotropes (e.g. adrenaline, dobutamine) directly affect cardiac performance and have a variable effect on systemic vascular resistance. Vasopressors (e.g. noradrenaline) increase vascular tone and systemic vascular resistance and have relatively little direct effect on cardiac pump function. The pharmacological properties of the commonly used agents are listed in Table 9.6.
| Inotrope | HR | SVR | MAP | CO |
|---|---|---|---|---|
| Dobutamine | ++ | ± | ± | ++ |
| Noradrenaline | − | ++ | ++ | ± |
| Adrenaline | + | + | + | ++ |
The principal goal of inotropic or vasopressor support is to improve tissue perfusion and restore normal aerobic metabolism. This is achieved by manipulating cardiovascular physiology in a way that seeks to reverse the processes that have resulted in shock. The differing haemodynamic profiles of the agents available allow some tailoring of inotropic support to the presumed mechanism(s) of shock.
Adrenaline
Adrenaline (epinephrine) may be used in patients with multifactorial shock, e.g. sepsis in a patient with heart failure. It causes both an increase in cardiac contractility and output (β 1 inotropic effect) and a moderate increase in SVR (SVR increased by α 1 stimulation, slightly offset by β 2 stimulation in skeletal muscle beds).
Dobutamine
Dobutamine is commonly used in patients with cardiogenic shock because it increases myocardial contractility and cardiac output (β 1 effect) but has little effect on SVR. β 2 stimulation in skeletal muscle beds can lead to significant vasodilatation in patients with sepsis in whom it can exacerbate hypotension and should be avoided.
Noradrenaline
Noradrenaline (norepinephrine) is a vasopressor. It is commonly used in patients with distributive forms of shock, e.g. septic shock. It results in peripheral vasoconstriction and an increase in SVR (α 1 effect). This increased afterload is beneficial in pure distributive shock but can adversely affect cardiac output in patients with significant LV dysfunction.
Outcome of shock
The mortality rate in septic shock is high and ranges from 30% to 50%. The prognosis of cardiogenic shock is poorer still. The outlook is best in hypovolaemic shock, assuming the cause of fluid or blood loss can be identified and treated.
Anaphylaxis
Anaphylaxis is an acute life-threatening reaction, triggered by an immunological mechanism and resulting in a release of histamine and other vasoactive mediators. Symptoms usually develop within minutes of exposure to the stimulus, but may be delayed by up to 30 min. They may include: cutaneous features (e.g. pruritus, flushing, urticaria, angio-oedema); gastrointestinal features (e.g. nausea, vomiting or diarrhoea); wheeze and respiratory distress; vasomotor symptoms (e.g. syncope, hypotension, dizziness, tachycardia).
Management of an acute attack
An ABCDE approach should be adopted (see ‘Assessment of the acutely ill patient’, p. 8). If the patient is shocked, proceed as suggested on p. 251. The patient should be laid flat with their legs elevated. Give adrenaline (epinephrine) 1:1000, 0.2–0.5 mL IM or SC; this can be repeated after 5 min (in children 0.01 mg/kg, maximum 0.3 mg). Inhaled adrenaline can also be used for laryngeal oedema. Chlorphenamine (10–20 mg IV) should also be given. In prolonged reactions, or in the presence of hypoxaemia, oxygen should be given. In patients with bronchospasm not responsive to adrenaline, nebulized salbutamol 5 mg should be used. Corticosteroids should be started in all patients: IV hydrocortisone (100 mg every 6 h) should be used in severe attacks; oral prednisolone (30–40 mg) can be used in less severe episodes. If the patient is taking β-blockers, glucagon should be given (1–5 mg IV over 5 min ± infusion). Severe or prolonged cases should be managed in an HDU setting. A reaction that has been successfully treated may recur up to 8–12 h later, and patients should remain as inpatients for at least this period to allow adequate monitoring.
Attempts should be made to prevent future attacks and distinguish between an anaphylactic reaction and disorders that may mimic anaphylaxis. Identify possible precipitants from the history: drugs, bites or stings, foodstuffs (especially nuts, shellfish, packaged food dyes), skin contacts (e.g. latex), preceding activities (e.g. exercise, seminal fluid). RAST, skin testing and specific challenge tests can be used to identify clinically relevant allergens, under specialist supervision.
Patients with confirmed anaphylaxis should be given education on the avoidance of possible future allergens, the wearing of MedicAlert® jewellery, and when and how to self-administer pre-loaded adrenaline injection syringes. In some patients, specialist allergen immunotherapy or desensitization can be helpful (e.g. in insect venom anaphylaxis).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


