Chapter 28 Sentinel lymph node biopsies
Introduction
This chapter is based on the author’s previous publications on this topic.1–4
Until recently, the treatment options were:
In an effort to resolve this dilemma, Morton, Cochran, and colleagues at UCLA developed the techniques of lymphatic mapping (LM) and sentinel node biopsy (SNB).5
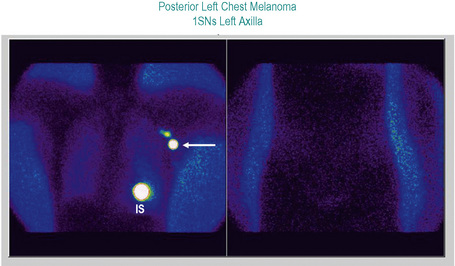
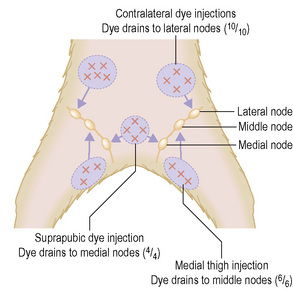
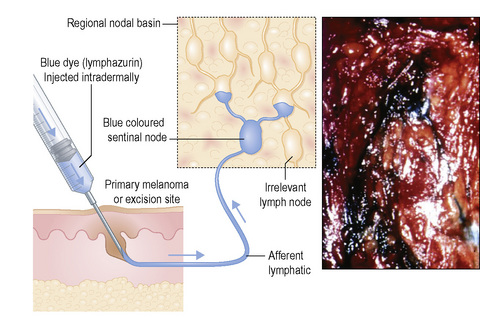
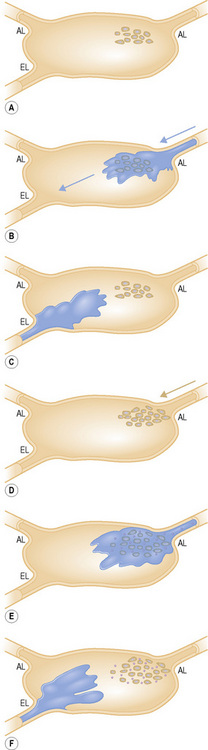
Studies of melanoma patients early in the evolution of regional nodal tumor spread showed that most had metastatic melanoma in a single node, the remaining lymph nodes in the regional basin being free of tumor.6 From this observation, we hypothesized that if it were possible to identify the individual node that was susceptible to these earliest metastases for subsequent surgical excision and histological scrutiny it would be possible accurately to stage the regional basin on the basis of a relatively minor surgical procedure. Lymphoscintigraphy, in which a radioactive isotope, such as 99mTc antimony sulfide, is injected around the site of a primary tumor, reliably identifies the lymph node basin(s) that receive lymph from that specific site.7 If the lymphoscintigram is read ‘early’ it is possible to identify the individual lymph node that first receives the isotope (Fig. 28.2).8 The location of that node can then be marked on the overlying skin. Animal experiments had previously shown that marker dye injected in anatomically definable areas of the skin drained reliably to a specific lymph node (Fig. 28 3).9 Combination of lymphoscintigraphy and intraoperative insertion of vital dye and isotope permitted the reliable identification of the first lymph node on the direct lymphatic drainage pathway from a primary melanoma, which, because it guards the remaining lymph nodes of the group, we called the sentinel node (SN) (Fig. 28.4).1 In a study of 223 melanoma patients who had a sentinel node biopsy followed by completion lymph node dissection (CLND) regardless of whether or not the SN contained melanoma, we showed that, if the SN was tumor free there was a less than 1% chance that nonsentinel nodes (NSN) would contain metastases. If the SN contained tumor there was a 16% chance that NSN would contain metastases.1 These findings lead to the development of an NIH-funded international randomized clinical trial (MSLT-I) in 2001. This compared (1) patients with intermediate thickness primary melanoma (1 mm and thicker or Clark level IV of any thickness), without clinically detectable regional or disseminated metastatic melanoma, who had wide excision and sentinel node biopsy with immediate CLND if the SN contained tumor, followed by observation, with (2) CLND only when the patients developed clinically detectable metastases during observation. Interim analysis of the results of MSLT-I has shown that SNB is a low-morbidity procedure and is the most accurate staging technique available for melanoma.10 Patients treated by SNB with immediate CLND if the SN contained tumor had significantly longer disease-free survival relative to observed patients. Patients who had CLND delayed until nodal metastasic disease became clinically detectable had significantly more tumor-positive nodes, were staged at a higher AJCC level, and had a significantly less favorable survival than patients with tumor in the SN who had an immediate CLND.11
Laboratory management and gross evaluation of sentinel nodes
If many lymph nodes are submitted, the specimen is probably the product of a partial regional lymph node dissection rather than a true SN biopsy (SNB). SNB, as originally described, provides a limited specimen that contains critical metastasis-susceptible nodal tissue, permitting a more detailed examination than is practicable for the multiple lymph nodes included in lymphadenectomy specimens.1 Surgeons need to be aware that there are significant labor and expense issues when sentinel node evaluation is required for specimens that contain multiple lymph nodes
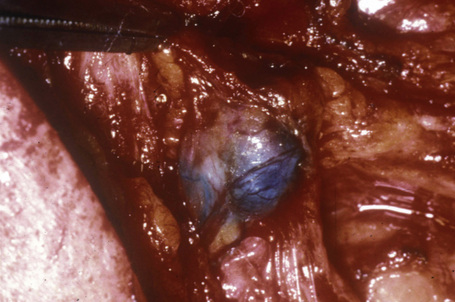
SN specimens are most informative if they are free of artifacts due to crush or cautery. SN may be partially dissected free of associated fat by the pathologist to facilitate processing, but it is good practice to leave a rim of fat so that entering lymphatics can be assessed for the presence of tumor. During dissection, the SN should be examined for blue coloration (Fig. 28.5) (although dye has usually dispersed by the time the specimen arrives at the laboratory). SN are measured (maximum length × width × thickness in millimeters) and exactly bisected through the longest meridian because melanoma cells first reach the subcapsular sinus via the afferent lymphatics which mostly enter the node in the plane of the central meridian.2 Cut surfaces are scrutinized for metastases, collections of melanin or carbon pigment (from tattoos or introduced by the surgeon). This inspection is improved by use of a hand lens or dissecting microscope. Imprints can be prepared at this stage for cytological evaluation (see below). The SN halves are placed cut face down in cassettes and fixed in formalin (Fig. 28.6), ideally for 12–24 hours, although a lesser period may be acceptable if the specimen arrived at the laboratory in an appropriate volume of formalin. Larger nodes may need to be cut more extensively, but additional slices should be cut parallel to the initial cut and parallel to the meridian.
Confirmation of the sentinel status of submitted lymph nodes
Correct identification of the SN is primarily the responsibility of the nuclear medicine physicians and surgeons. Because of technical problems during nuclear medicine and surgical procedures, lymph nodes submitted as sentinel may not be true SNs. Tumor within a sentinel node can obstruct and divert lymph flow to another node, leading to incorrect designation of that node that contains blue dye and shows enhanced radioactivity as sentinel. Preoperative ultrasonography can identify nodal metastases larger than 5 mm (and some claim that smaller deposits are detectable by ultrasound.1,2 If putative tumor deposits detected by ultrasound are confirmed as melanoma by fine needle aspiration, the patient may be considered for completion lymph node dissection instead of SNB.
Carbon particles injected intradermally with blue dye accumulate preferentially and usually exclusively in SN.3 Particles accumulate in subcapsular sinuses and lymphoid tissues around the entry point of afferent lymphatics (Fig. 28.7). The location of these particles fortuitously indicates the area of the lymph node most likely to harbor metastatic tumor cells, since the carbon particles are delivered to the SN via the same lymphatics as tumor cells shed by the primary melanoma.4 This approach cannot be used in patients with carbon-based permanent black tattoos of skin in the catchment area of the SN because tattoo pigment frequently tracks to regional lymph nodes. Drug regulations vary from country to country and widespread use of this interesting and potentially valuable technique must await the clearance of substantial regulatory hurdles.
In Australia, antimony sulfur colloid is routinely used for lymphoscintigraphy prior to SN biopsy.5 Scolyer and coauthors report the use of inductively coupled plasma mass spectrometry of tissue sections to confirm SN status by detection of increased amounts of antimony: however this approach is still in development and is not presently available for routine use.6
Can tissue from a sentinel node ethically be made available for research?
Accurate determination of the presence or absence of tumor in the SN is essential for correct staging of patients and to allow planning of optimal management. Identification of tumor status of an SN may be difficult as the amount of tumor present may be very small and occupy a limited area of the SN. Underassessment of the SN has serious consequences. Patients who develop ipsilateral regional nodal metastases after a reportedly tumor-free SN (false-negative SN) have an outcome that is at least as bad and possibly worse than patients who develop clinically detectable metastases in the regional nodes during observation after wide excision.1 Not all false-negative SN can be attributed to pathologist error. Some are due to misidentification of a nonsentinel node as sentinel during lymphoscintigraphy or at surgery. Others reflect vagaries of the biology of melanoma spread. For example, while at the time of SNB there may be no tumor cells visible in the sentinel node or at the excised primary site, clinically undetectable tumor cells present in the afferent lymphatics may arrive in the nodal basin after the SNB has been performed, seed in the remaining lymph nodes, and with time grow to clinically detectable size. Pathologists should therefore be extremely cautious in providing SN tissue to investigators until after the SN tumor status has been established.
Intraoperative evaluation of sentinel nodes
Early in our development of LM/SNB we evaluated the SN by intraoperative frozen sections and rapid immunohistochemistry to make possible immediate completion lymph node dissection if the SN contained tumor.1 This had the advantage of sparing patients a second anesthetic and surgical procedure. However, experience has shown that evaluation of SN from melanoma patients on the basis of intraoperative frozen sections is relatively unreliable. Preparation of a full-face frozen section is necessary to allow complete evaluation of the subcapsular sinus where early tumor is often detected. To obtain a section that includes the entire subcapsular sinus from a frozen block often requires that many of the initial sections are discarded, leading to loss of substantial nodal tissue. Since SN metastases are frequently very small and selectively located in the area of the nodal meridian, the limited diagnostic tissue may be entirely lost with these initial discarded sections. Additionally, identification of single melanoma cells or small clusters of melanoma cells or nevocytically differentiated melanoma cells is more difficult in frozen sections than in well-stained slides from fully fixed tissues. We and other authors strongly believe that melanoma-draining SNs should be evaluated using thin sections cut from well-fixed paraffin-embedded tissues.2,3 If intraoperative assessment is requested, visual assessment of the cut face of the node for deposits of tumor is recommended, using a hand lens or dissecting microscope if necessary. Cell smears may be obtained by scraping the nodal cut surfaces, or tumor imprints prepared by pressing the cut surfaces of SN onto glass slides for cytological evaluation (Fig. 28.8). Interpretation of such preparations can be challenging if the melanoma cells are few in number or small and nevocytically differentiated and the opinion of an experienced cytopathologist is likely to be necessary.
The need to evaluate multiple levels of the sentinel node
Sectioning
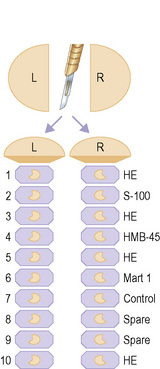
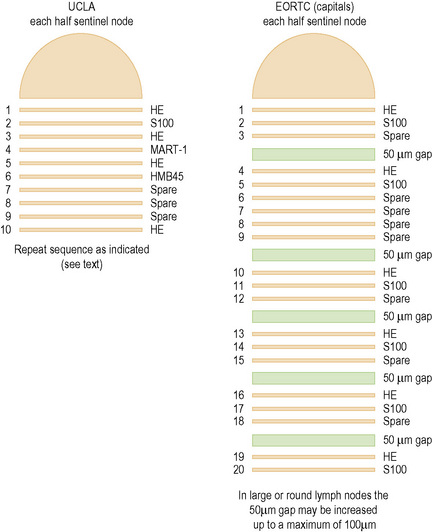
In initial studies of nodal micrometastases, we showed that early melanoma metastases are usually located in a relatively narrow band of tissue adjacent to the longest nodal meridian.1 Thus carefully sampling the tissues adjacent to the nodal meridian should detect the subgroup of melanoma patients with early nodal metastases. In this study, additional sampling of more peripheral areas of the node did not increase the proportion of melanoma-positive lymph nodes. This is in contrast to other tumors, such as breast cancer, where the tumor cells spread more widely across the nodal surface and thus more extensive sampling progressively increases the proportion of tumor-positive lymph nodes. On the basis of these findings in lymph nodes from melanoma patients, we have consistently recommended examination of 10 full-face serial sections cut from both faces of the node and stained by H&E (sections 1, 3, 5, and 10), S-100 (section 2), HMB-45 (section 4) and MART-1 (section 6).1 Sections 7–9 are retained as spares. If tumor cells are not found in the initial twenty sections cut from both halves of a SN of a patient with a primary melanoma thicker than 1.2 mm (and thus significantly at risk for nodal metastases), additional sections may be prepared and examined, although the yield from this additional evaluation is, in our experience, low. This approach detects melanoma in 16–20% of SNB specimens, a figure that varies according to the thickness of the primary melanomas included in the study group and is closely similar to the rate of development of clinically detectable metastatic melanoma in the ipsilateral regional lymph nodes of patients observed for up to 10 years after wide excision of a primary melanoma.2 It is arguable that this relatively simple focused sampling protocol detects most or all of the clinically significant melanoma deposits in SN.3
Recent studies, however, have reported that more extended sampling to allow evaluation of sections cut from deeper areas of SN identifies melanoma metastases in some patients where sections closer to the nodal meridian were tumor free.4–8 The clinical significance of these additional melanoma cells detected by extended sampling remains unknown and elucidation of their biology will require that patients be followed for up to 10 years.
Cook and coworkers have reported that examination of six pairs of sections cut at 50-µm intervals and stained respectively with H&E and S-100 detects melanoma in up to 33.8% of SNs.5 Spare sections are cut at each level and retained to permit additional immunohistochemistry in the event of ambiguous results from the initial sections. These studies were undertaken because the authors had encountered practical difficulty in accurately cutting the SN through the true meridianal plane and wished to reconcile reported differences in the rate of detection of tumor-positive SN by histopathology and molecular techniques (RT-PCR) (see below). A modification of this protocol has recently been adopted by the European Organization for Research and Treatment of Cancer (EORTC) and is currently a requirement for evaluation of the SN of melanoma patients entering EORTC clinical trials (Fig. 28.9).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree