and Alena Skalova2
(1)
Departamento de Ciências Biomédicas e Medicina, Universidade do Algarve, Faro, Portugal
(2)
Department of Pathology, Medical Faculty Charles University, Plzen, Czech Republic
11.1 Definition, Site and Incidence
Salivary duct carcinoma (SDC) is an aggressive primary salivary malignancy which microscopically resembles high-grade ductal carcinoma of the breast, with both in situ and invasive patterns. It is typically found in older men, most often in the parotid. It can arise de novo or as the malignant component of carcinoma ex pleomorphic adenoma [75]. Previously thought to be extremely rare, it is now recognised as not infrequent and accounts for up to 2 % of all primary salivary epithelial neoplasms and 7–10 % of malignant salivary gland tumours. In approximately one-tenth of cases, it constitutes the most common carcinoma component in carcinoma ex pleomorphic adenoma [4, 17, 31, 44, 49, 56]. On exceedingly rare occasions, SDC may arise in other salivary gland neoplasms, such as polymorphous low-grade adenocarcinoma [77] and Warthin tumour [51], or in a background of IgG4-related autoimmune disease of the parotid gland [28]. Salivary duct carcinoma also occurs as part of hybrid carcinomas (see Chap. 15). As emphasised in Chap. 7 and elsewhere, due to a number of reasons, the relative frequency of an individual salivary gland tumour is extremely difficult to estimate with accuracy. Salivary duct carcinoma is in many studies the fifth most common malignant tumour after mucoepidermoid carcinoma, adenoid cystic carcinoma, acinic cell carcinoma and carcinoma ex pleomorphic adenoma. These five tumours constitute 85–90 % of all salivary malignancies.
Salivary duct carcinoma is an aggressive adenocarcinoma with striking histological resemblance to high-grade ductal breast carcinoma and was first described in 1968 by Kleinsasser and associates [52]. The histology was further refined by Garland and associates in 1984, and SDC was recognised as a separate entity in the second WHO classification in 1991 [27, 74]. Since the report by Kleinsasser and associates, a large number of case reports and smaller series have been published, each one consisting of a dozen or so of cases of SDCs [1, 6, 13, 18, 23, 24, 26, 31, 34, 37, 39, 48, 58, 76, 82, 83, 86, 89, 100]. In later years, there have also been a number a larger series published, and the largest cohort of SDCs to date consisting of 84 cases from the M.D. Anderson Cancer Center was published by Williams and associates in 2007 [25, 32, 44, 56, 57, 72, 94]. Salivary duct carcinoma is mainly a parotid tumour with 78–85 % located in the parotid gland, 12–13 % in the submandibular gland and 2–10 % in the minor salivary glands. A rare case arising in the extraglandular segment of Stensen’s duct has also been reported [67]. A literature review of intraoral SDCs by Huh and associates in 2003 indicated that intraoral SDC most frequently occurs in the palate (approximately half of cases) followed by the buccal mucosa [38]. Salivary duct carcinoma is primarily a tumour of elderly men (male to female ratio 4–5:1), and many cases occur in the sixth or seventh decades [44, 94]. Most reports indicate that SDC is a highly malignant tumour with poor clinical outcome. Other high-grade salivary gland tumours, e.g. adenoid cystic carcinoma, tend to have late recurrences and metastases and are highly malignant on a long-term basis (10–20 years), whilst SDC seems to have the most dismal short-term prognosis of all salivary gland tumours [6, 39, 57, 60].
Whereas invasive ductal carcinoma and ductal carcinoma in situ of the breast have been intensively studied, it is only during recent years it has become apparent that histological variants of salivary duct carcinoma also exist. At present, three histological variants have been described. These are a sarcomatoid type of SDC with an admixed sarcomatoid component, a mucin-rich variant with features of mucinous/colloid carcinoma and an invasive micropapillary variant of SDC. The significance of recognising these subtypes remains to be elucidated. Another variant of SDC, or perhaps indeed a separate entity, is the important concept of intraductal carcinoma or by some called low-grade SDC (and other names). The nomenclature of this variant is still rather controversial. Furthermore, it has also been demonstrated that SDCs comprise phenotypically and genotypically diverse neoplasms [40]. The histological recognition of the different subtypes is warranted as some have a more favourable clinical course, and different therapeutic strategies may develop in the future as more knowledge is accumulated.
11.2 Gross Appearance
Grossly, SDC is usually a firm ill-defined mass infiltrating the surrounding gland and soft tissue. A well-circumscribed nodule within the tumour may indicate a pre-existing pleomorphic adenoma. Salivary duct carcinomas are primarily parotid tumours and typically present as rapidly growing swellings, often associated with pain and facial paresis and frequently present with Stage III or IV disease. A large proportion of patients (66–78 %) present with a T3/T4 tumour, i.e. more than 4 cm in size, and in more than 50 % of cases, cervical lymph node metastases are clinically present already at the time of diagnosis [44, 94]. Parotid tumours typically present as a firm, white, tan or grey mass which may be partly circumscribed or multifocal (Fig. 11.1).
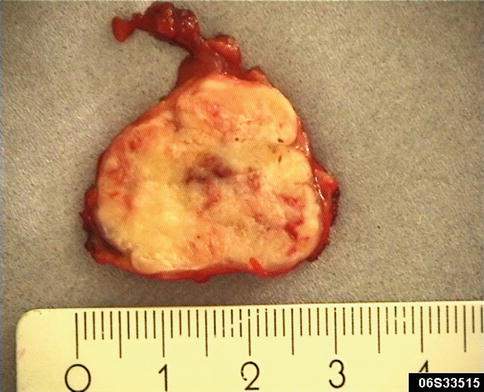

Fig. 11.1
Gross appearance of SDC as a firm mass with whitish cut surface and central cystic area. This tumour is smaller than average, measuring only approximately 3 cm
11.3 Histopathology
Salivary duct carcinoma is characterised by pleomorphic cells that form aggregates resembling distended ducts and solid nests. Salivary duct carcinoma consists of relatively large, polygonal usually eosinophilic cells that most likely derive from the excretory ducts. The frequently eosinophilic cells, often with apocrine differentiation, show nuclear pleomorphism, hyperchromasia and frequent mitoses. The cells may contain PAS-positive, diastase-sensitive granules. Mucicarmine and alcian blue stains are usually negative. In its typical form, SDC displays combinations of solid, cribriform, papillary and comedo patterns, embedded in a desmoplastic, often hyaline, stroma. The most striking features are cribriform growth patterns and in situ formations similar to those seen in ductal breast lesions (Fig. 11.2). The intraductal component is papillary and cribriform or shows a solid growth pattern, often with comedonecrosis. The invasive component consists of irregular glands and cords of cells that characteristically elicit a pronounced desmoplastic reaction, as seen in invasive mammary ductal carcinoma. The desmoplastic stroma is however not always present, but the infiltrating desmoplastic component is a predominant feature in most SDC (Fig. 11.3). Occasionally, there is a diffuse growth of single cells and small ill-defined clusters. Ductal carcinoma in situ extending within the lobules of the parotid gland (cancerisation of lobules) is often present. The in situ lesions can be masked by extensive growth of invasive carcinoma and are morphologically subtle, although they can be highlighted by using basal-myoepithelial markers [19]. Calcification and psammoma bodies are frequent findings, whilst sebaceous metaplasia is rare (Fig. 11.4). SDC may also show papillary growth patterns with or without psammoma bodies, and the cells here tend to be pleomorphic with eosinophilic, granular or oncocytic cytoplasm (Fig. 11.5). Ductal carcinoma in situ extending within the lobules of the parotid gland (cancerisation of lobules) is often present. The cellular pleomorphism and mitotic rate vary. The cells tend to be rather large and polygonal, somewhat eosinophilic and with a moderate to pronounced nuclear atypia. In other cases, the cells are more undifferentiated with vesicular nuclei and prominent nucleoli. In yet other areas, the cells may have abundant rather clear cytoplasm with microvesicles (Fig. 11.6). Mitotic figures are often frequent, and perineural spread is seen in approximately 60 % of cases and vascular invasion in 30 % [4] (Fig. 11.7). Comedonecrosis is almost invariably present (Fig. 11.8). The histology is clearly that of a high-grade malignancy. The histological features of cribriform and in situ growth patterns, fibrous/hyaline stroma, comedonecrosis, cytological atypia and invasive growth together with a high proportion of cases positive for androgen receptor (AR) and to a lesser degree for HER2 (HER2/neu) really exclude any other primary salivary gland tumour. In cases where SDC arises as the malignant component in a carcinoma ex pleomorphic adenoma, evidence of previous or coexistent pleomorphic adenoma is most often seen as separate, frequently sclerotic nodule.
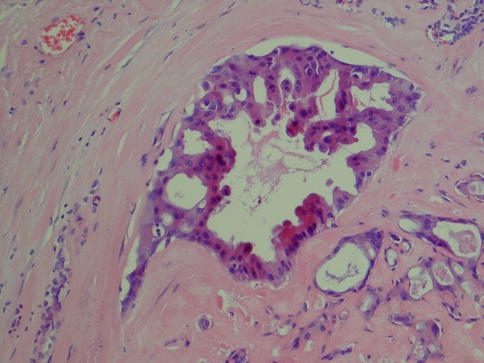
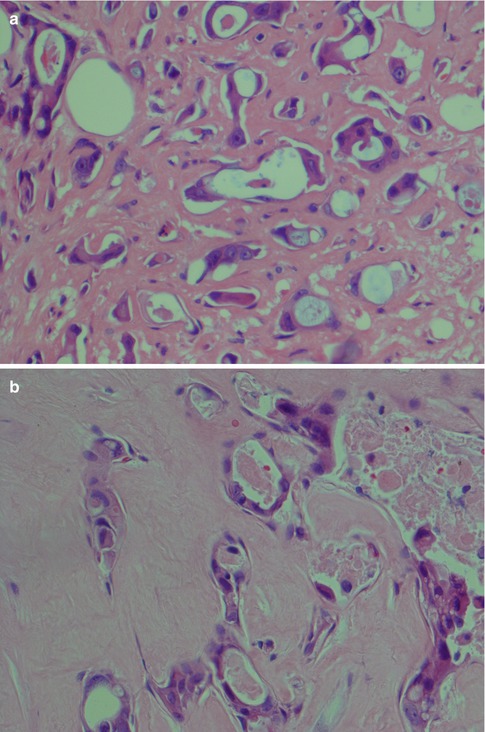
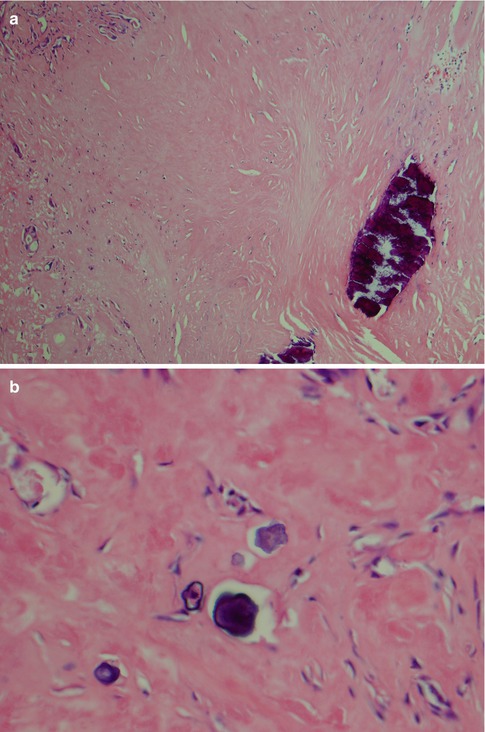
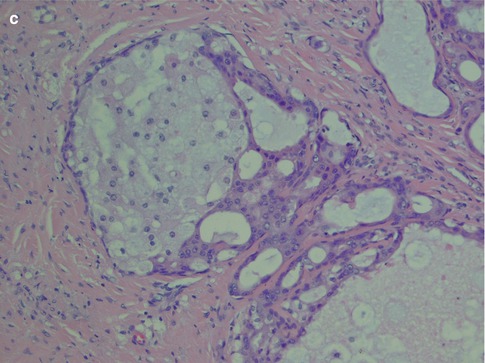
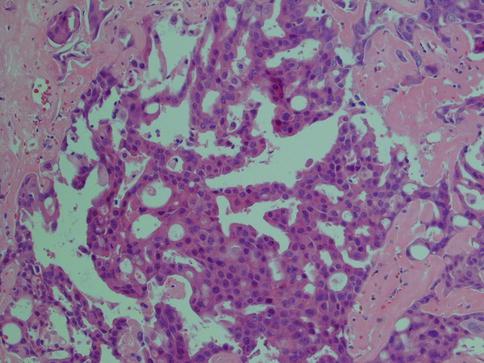
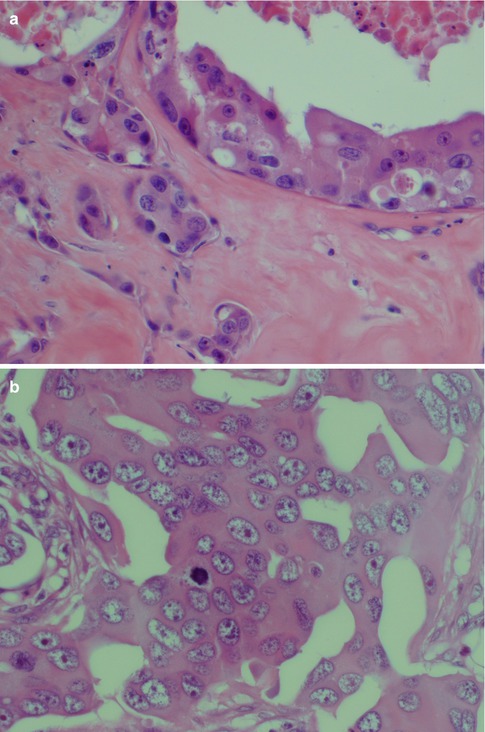
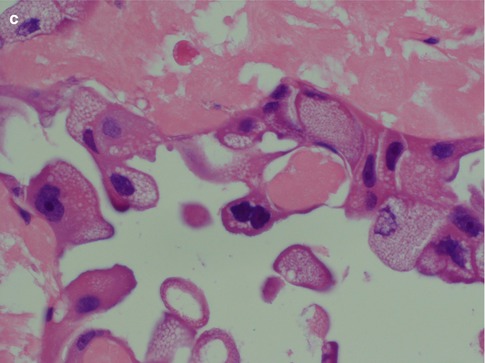
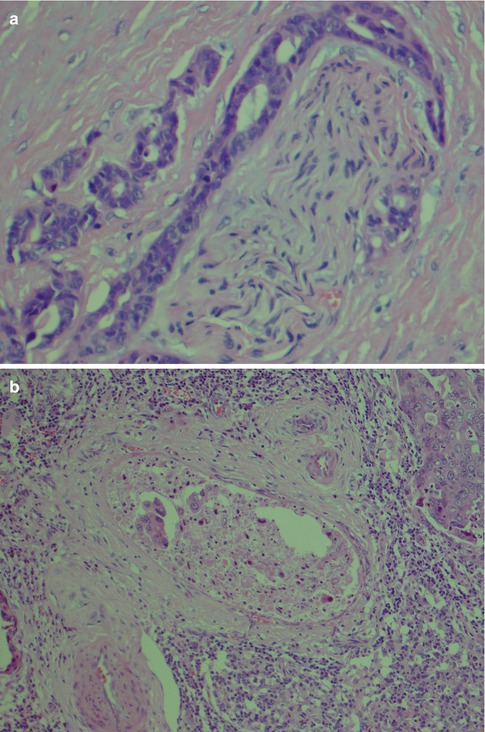
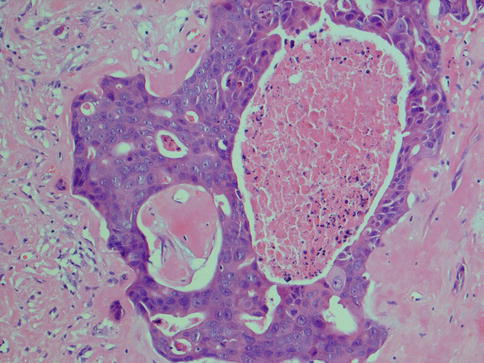

Fig. 11.2
Salivary duct carcinoma. Typical intraductal cribriform growth pattern but also invasive growth (right)

Fig. 11.3
(a) Microglandular infiltration in a fibrous stroma. (b) Invasive ductal cells in a hyaline sclerotic stroma


Fig. 11.4
(a) Calcification in a dense hyaline stroma. (b) Psammoma bodies in a fibrous/hyaline stroma. (c) Sebaceous metaplasia

Fig. 11.5
SDC with papillary growth pattern and oncocytic cells


Fig. 11.6
(a) Typical eosinophilic ductal cells with moderate atypia. (b) A more undifferentiated appearance with vesicular nuclei, many with prominent nucleoli. (c) Ductal cells with abundant cytoplasm that contain microvesicles

Fig. 11.7
(a) Perineural invasion as well as intraneural growth (right). (b) Vascular invasion

Fig. 11.8
SDC with comedonecrosis
Several histological variants of SDC have been described. The full clinical implication of a histological subclassification of SDC remains to be seen, but sarcomatoid, mucin-rich and invasive micropapillary variants have been reported in the literature. Also a pure in situ form of SDC, known as intraductal carcinoma (low-grade SDC), has been described albeit the terminology still is controversial (see below).
11.3.1 Immunohistochemistry
Immunohistochemically, SDC is positive with broad-spectrum and low molecular weight cytokeratins and epithelial membrane antigen (EMA). It is also strongly and diffusely positive with CK7, and there is occasionally focal staining with CK20 [65]. It is also positive for CEA and to a lesser degree for GCDFP-15 and CD44 (Fig. 11.9). It is typically negative with S-100 protein and basal-myoepithelial markers, such as cytokeratins 5/6 and 14, p63, calponin and smooth muscle myosin heavy chain (SMMHC), although these highlight surrounding non-neoplastic cells of in situ lesions. In most examples of SDC, the MIB1 proliferative index is over 25 %.
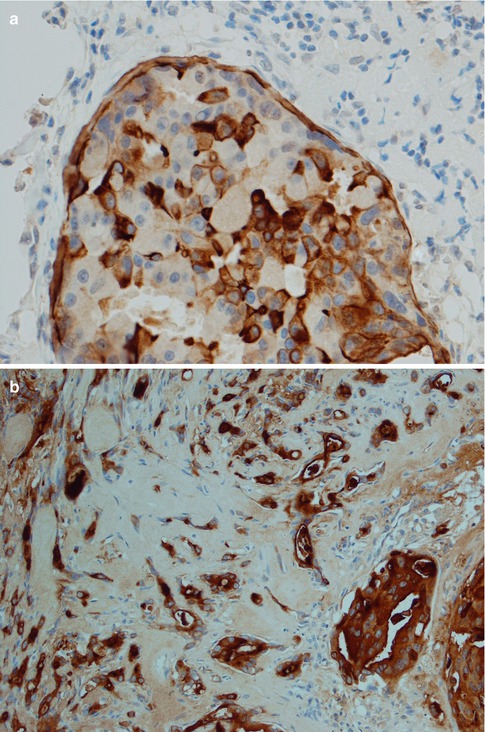
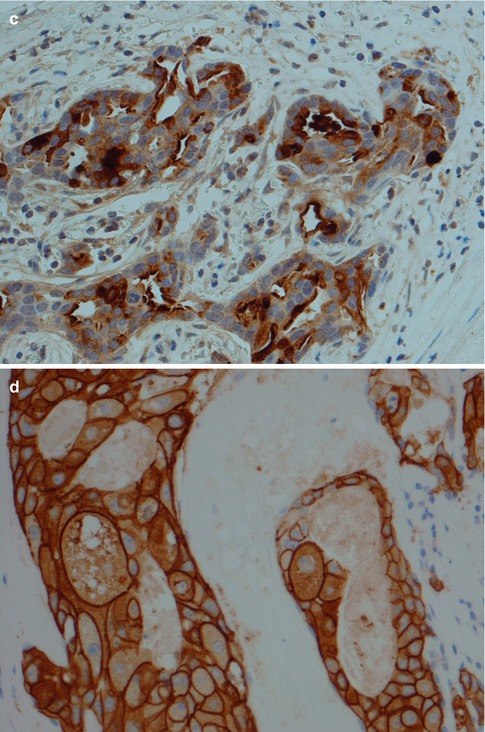
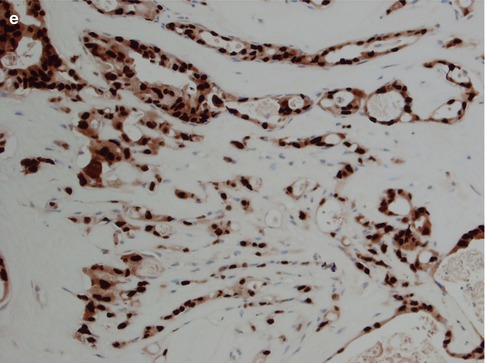



Fig. 11.9
Immunohistochemistry of SDC. (a) CK7. (b) EMA. (c) Focal positivity for GCDFP-15. (d) SDC with strong HER2 staining. (e) Strong nuclear staining with AR
Salivary duct carcinoma has previously been regarded as almost invariable androgen receptor (AR) positive [9, 48, 56, 78] and to a very high extent also HER2 positive [29, 34, 58, 82, 83]. In recent years, however, larger studies have shown a somewhat different picture that cannot be explained by possible inclusion of variants of SDC (e.g. intraductal carcinoma) in their series. It may also be worth mentioning that an earlier study from 1992 indicated that positive HER2 staining was seen inasmuch as 6 of 20 salivary adenocarcinomas and in 7 of 15 carcinoma ex pleomorphic adenomas, but in none of the remaining 24 cases of adenoid cystic carcinoma, mucoepidermoid carcinoma and squamous cell carcinoma [84]. In one study of 84 SDCs, 67 % expressed AR and only 25 % expressed high levels of HER2. Co-expression of AR and HER2 was seen in only 17 % of tumours [94]. In yet another series, 34 cases of SDC were investigated and only 47 % were HER2 positive [44]. The previous prevailing opinion that SDCs express AR and HER2 to a very high degree is therefore no longer accurate. Salivary duct carcinomas frequently do express AR and HER2 but not to a very high degree. In the large study by Williams and associates, the most commonly expressed of the hormonal and growth factor receptors were PELP1 (proline-, glutamic acid- and leucine-rich protein-1), ERβ and AR. These receptors were expressed in 94, 73 and 67 % of cases, respectively. PELP1 is a novel oestrogen receptor coactivator, also called the modulator of nongenomic activity of ER (MNAR). Epidermal growth factor receptor (EGFR) and HER2 were overexpressed individually in 48 and 25 % of cases, respectively, and co-overexpressed in 12 % [94]. It is yet not known to what extent PELP1 is expressed in other salivary gland carcinomas, but PELP1 may assist not only in finding novel therapies for patients with SDC but may also act as a potential ancillary diagnostic marker for SDC (Tables 11.1 and 11.2).
Table 11.1
Common immunohistochemical staining properties of salivary duct carcinoma with commonly available antibodies
Usually positive staining | Most keratins |
PELP1a | |
ERβ | |
AR | |
GCDFP-15 | |
Variably positive staining | HER2 |
EGFR | |
EMA | |
CEA | |
GCDFP-15 | |
CD44 | |
Usually negative staining | CK20 |
p63 | |
SMA | |
S-100b | |
Vimentin | |
C-kit | |
ER, PR |
Table 11.2
Hormonal and growth factor-related markers in salivary duct carcinoma
Hormonal biomarkers | |
PELP1 | 94 % |
ERβ | 73 % |
AR | 67 % |
Co-expression of all 3 | 45 % |
Co-expression of 2 of the 3 | 81 % |
Expression of 1 only | 8 % |
Negative | <1 % |
Growth factor-related markers | |
HER2 (3+) | 25 % |
EGFR (3+) | 48 % |
Co-expression of both | 14 % |
11.3.2 Histological Variants of Salivary Duct Carcinoma
Currently three histological variants of SDCs are known, namely, sarcomatoid SDC, mucin-rich SDC and invasive micropapillary SDC [14]. The full clinical implication of a histological subclassification of SDC remains to be seen, but sarcomatoid, mucin-rich and invasive micropapillary variants have been reported in the literature. Also a pure in situ form of SDC, known as intraductal carcinoma (low-grade SDC), has been described albeit the terminology still is controversial (see below). Foci of classic SDC are present, and therefore these variants will usually not pose any major diagnostic problem.
11.3.2.1 Sarcomatoid Salivary Duct Carcinoma
Sarcomatoid SDC has as implicated by its name an admixed sarcomatoid component (Fig. 11.10). The histology and nomenclature of such tumours have been a matter of debate for many years. Sarcomatoid SDC does however seem more appropriate than the term carcinosarcoma as immunohistochemical and molecular studies of these and similar biphasic tumours imply a monoclonal origin [68]. The exception may possibly be carcinosarcoma arising in pleomorphic adenoma, so-called true malignant mixed tumour. In their literature review in 2004, Nagao and associates [62] identified 11 cases of sarcomatoid SDC, albeit only four of which had been reported as sarcomatoid SDC, the others as carcinosarcoma [2, 5, 10, 30, 35, 41, 69, 81, 85]. Nagao and associates added 8 cases of their own, and since then another 4–5 cases have been reported [46, 59, 68, 87], making the total number of reported sarcomatoid SDC to some 20–25 cases only.
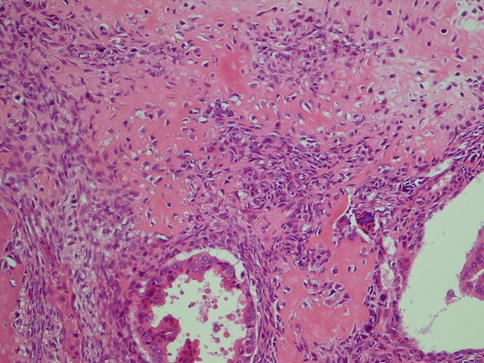

Fig. 11.10
Sarcomatoid SDC with an osteocartilaginous sarcomatous component
11.3.2.2 Mucin-Rich Salivary Duct Carcinoma
The mucin–rich variant of SDC is an even rarer variant than the sarcomatoid SDC with features of mucinous/colloid carcinoma in which clusters of carcinoma cells float in mucin pools. Since the first four cases were reported by Simpson and associates in 2003 [78], only five additional cases have been described in the English literature [36, 97]. Although these nine cases showed typical demographics of conventional SDC with regard to age and sex (older males), the typical parotid location is not that obvious. The vast majority of conventional SDCs arise in the parotid gland, whilst only two of six mucin-rich variants did. Two cases arose in the floor of mouth, one in the palate (in a pre-existing pleomorphic adenoma) and one in the submandibular gland. One may also speculate, on the ground of its rarity, that some mucin-rich SDC could represent a composite tumour of SDC and mucinous carcinoma. This may perhaps be further supported by a case of mucin-rich SDC where the central portion of the tumour histologically was an osteoclast-type giant cell tumour [54]. Extracellular mucin in small quantities is not an uncommon finding in salivary duct carcinoma, but the presence of lakes with mucin-containing malignant cells is hence very rare. Simpson et al. described the colloid carcinoma element to comprise large mucin lakes divided into compartments by fibrous bands. The mucin lakes accounted for 40–50 % of total tumour area in three of the cases and in one case only smaller scattered pools of mucin accounting for approximately 2 % of tumour area. Within these mucinous pools, tumour cells ‘floated’ individually and in clusters. The colloid carcinoma elements were closely intermingled with areas of conventional SDC [78].
11.3.2.3 Invasive Micropapillary Salivary Duct Carcinoma
Although papillary growth pattern is frequently seen in the intraductal component of SDC, the invasive papillary counterpart was not formally recognised until recently. In 2004, Nagao and associates described 14 cases of SDC with an invasive micropapillary component [63]. This variant was termed invasive micropapillary SDC and is also recognised in the 2005 WHO classification [9]. Invasive micropapillary SDC is characterised by morula-like small cell clusters without fibrovascular cores surrounded by clear spaces. The cells have eosinophilic cytoplasm and vesicular nuclei and the surrounding clear spaces are devoid of mucus (Fig. 11.11). Since the report by Nagao et al., only one more publication is to be found in the English literature [98]. This paucity of reports is possibly not a reflection of the true incidence but perhaps merely that recent large studies on SDCs have put emphasis on immunoprofile, hormone receptor status, etc., and conventional descriptive histopathology has not been the primary object of these studies. The 14 cases reported by Nagao and co-workers [63] were retrieved from a total of 82 cases of salivary duct carcinoma, and hence invasive micropapillary SDC constituted 17 % of SDCs at that institute. Criteria for the diagnosis of invasive micropapillary SDC were identical to those for carcinoma of the breast established in 1993 [63, 80]. The study of Nagao and associates indicates that invasive micropapillary SDC is a highly aggressive variant of SDC which is in sharp contrast to intraductal carcinoma or low-grade SDC (see below). All 14 patients had regional lymph node metastasis at the time of the initial diagnosis, and 9 of the 14 patients died within 24 months of diagnosis. The tumours with invasive micropapillary foci were smaller than conventional SDCs with a mean size of 2.4 cm (conventional SDCs are usually larger than 3–4 cm) and behaved even more aggressively than conventional SDCs [63].
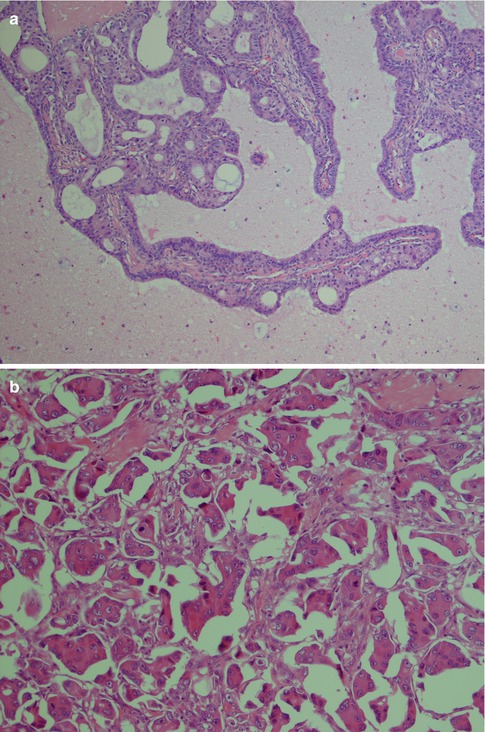

Fig. 11.11
(a) Unusual SDC with well formed papillae with fibrovascular cores. (b) A more typical micropapillary SDC with morula-like clusters without fibrovascular cores
11.3.2.4 Intraductal Carcinoma
Sarcomatoid, mucin-rich and invasive papillary variants of SDC are histologically recognisable, but the importance of this subclassification remains to be seen. The recognition of the so-called intraductal carcinoma (low–grade SDC, low–grade cribriform cystadenocarcinoma, salivary duct carcinoma in situ) is, on the contrast, very important as if completely excised, it appears to have a totally benign clinical course. It likely represents a precursor lesion to adenocarcinoma(s) and as such should be regarded as a premalignant lesion (Fig. 11.12). The terminology still is debated and controversial, but whatever name used it represents an important concept that needs further studies. There are numerous similarities between breast and salivary malignancies, and it is only to be expected that in situ lesions are present in the salivary glands as well. Salivary intraductal carcinoma was first described by Chen in 1983 and is defined by the presence of an in situ ductal carcinoma with intact myoepithelial cell layer around all tumour islands. One may argue that the surrounding cells are basal cells rather than myoepithelial cells should the origin of this tumour be the excretory duct where no myoepithelial cells are present and as basal cells share very much the same immunoprofile as myoepithelial cells. Intraductal carcinoma appears to represent a possible preinvasive phase of SDC [11, 15]. This tumour, or its mimic, has been reported as intraductal carcinoma [3, 8, 12, 50, 90, 92, 99] or under the designation low-grade salivary duct carcinoma [7, 17]. We tend to agree with the proposal of Cheuk and Chan [14] that the term intraductal carcinoma is more appropriate than low-grade salivary duct carcinoma as it emphasises the fundamental nature of tumour being purely in situ and entirely separated from the vastly more aggressive salivary duct carcinoma. This opinion has also been voiced by others [11, 15, 91, 92]. The low-grade cribriform cystadenocarcinoma described in the 2005 WHO classification is defined as ‘a rare, cystic, proliferative carcinoma that resembles the spectrum of breast lesions from atypical ductal hyperplasia to micropapillary and cribriform low-grade ductal carcinoma in situ’. As synonym, low-grade salivary duct is mentioned [8]. In 2008, yet another proposal was put forward by Simpson and co-workers [79], suggesting the term salivary duct carcinoma in situ. Simpson and co-workers point out that at the moment we do not know what possible relationship there is between SDC in situ and low-grade cribriform cystadenocarcinoma. They may represent the same thing or being two separate entities.
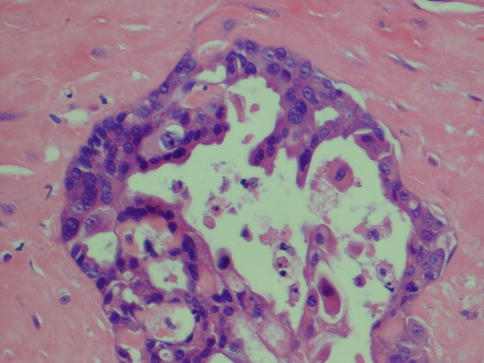

Fig. 11.12
Intraductal carcinoma
If this tumour ‘entity’ is indeed a precursor lesion to salivary duct carcinoma and to SDC only, the term salivary duct carcinoma in situ is to be preferred instead of intraductal carcinoma. Should, on the other hand, this in situ tumour also be capable to develop to any invasive malignancy other than SDC, the term intraductal carcinoma seems more appropriate. Arguments for the latter have been put forward by Ihrler and associates [42, 43]. In 15 of 22 cases of adenocarcinoma, not otherwise specified, Ihrler and associates [42] described foci of intraductal carcinoma. The tumour cells in the intraductal structures were CK14-positive, actin-negative ductal basal cells. None of these 22 cases hence showed features of salivary duct carcinoma. Their findings suggest that intraductal carcinoma possibly is a preinvasive precursor of adenocarcinoma, not otherwise specified. In another study by Ihrler and co-workers [43], 19 cases of carcinoma ex pleomorphic adenoma were investigated with regard to foci of intraductal carcinoma. Fifteen cases showed areas with intraductal carcinoma, eight of which were purely intracapsular carcinoma ex pleomorphic adenoma. Out of the remaining seven cases, five also showed extracapsular invasive carcinoma. At least two of the invasive carcinomas were not salivary duct carcinoma but adenocarcinoma NOS. Their studies support that intraductal carcinoma represents a preinvasive lesion not only for salivary duct carcinoma but for adenocarcinoma NOS and also a precursor in the malignant transformation of pleomorphic adenoma to carcinoma ex pleomorphic adenoma [42, 43]. It is also possible that part of the tumours in the ever-diminishing group of adenocarcinoma NOS are in fact examples of ‘triple-negative’ intermediate type of salivary duct carcinoma (see below). Still, it seems appropriate, at least for the moment, to keep the term intraductal carcinoma rather than low-grade salivary duct carcinoma or salivary duct carcinoma in situ.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


