Ruptured Abdominal Aortic Aneurysm
Frank Pomposelli
Jeffrey Kalish
Introduction
No emergency in vascular surgery is more acute or lethal than the rupture of an abdominal aortic aneurysm (AAA). Ruptured AAAs are the 15th leading cause of death overall and the 10th leading cause of death in men older than age 55 in the United States, claiming more than 15,000 lives annually. Although the true incidence of aortic rupture is difficult to determine, the mortality rate for the patients arriving to the hospital alive ranges from 40% to 70%. When available autopsy data are taken into account, including the patients who die before reaching the hospital, the true mortality rate is probably 90% or more. Although the advances in anesthetic and surgical techniques have reduced the perioperative mortality to 2% to 4% for elective repair of intact AAAs, the surgical mortality rates continue to average 50% for the ruptured AAA and have changed little in many years. Since aortic aneurysm is an insidious process rarely causing any symptoms prior to the rupture, the reduction in mortality for this condition depends primarily on timely recognition before the occurrence of rupture. Implementation of population-based screening for high-risk populations, such as the “Screening Abdominal Aortic Aneurysms Very Efficiently” (SAAAVE) Act which was instituted by Congress beginning in January of 2007, provides the best solution for reducing the occurrence of rupture. Under current Congressional guidelines, this one-time ultrasound screening is limited to the men who have smoked at least 100 cigarettes during their life, and men and women
with a family history of AAA as part of their Welcome to Medicare Physical Exam.
with a family history of AAA as part of their Welcome to Medicare Physical Exam.
Once rupture occurs, patient survival depends on prompt surgical intervention to control hemorrhage and aggressive and often complex postoperative critical care management. The development of endovascular aneurysm repair (EVAR) has radically changed the approach to elective repair of aortic aneurysm, reducing mortality, morbidity, and time to recovery for many patients treated by this approach. As experience with EVAR flourished over the past decade, many centers began applying this technique to the selected patients presenting with rupture. Increasing data continues to emerge suggesting that the rate of perioperative mortality is lower than the expected historical rate of 50% to 70%, offering the promise of better outcomes for the first time in more than four decades. This chapter reviews the diagnosis, treatment, postoperative care, and outcomes of contemporary treatment of ruptured AAA.
Diagnosis
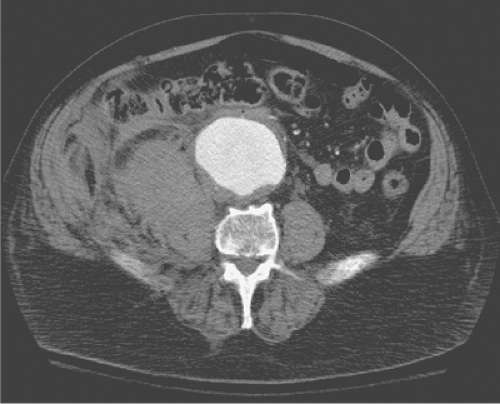
The identification of a patient presenting with ruptured AAA is first and foremost a clinical diagnosis based on a careful history and physical examination. The classic triad for ruptured AAA presentation is severe abdominal or back pain, hypotension, and a pulsatile abdominal mass. Patients presenting with this constellation of symptoms and findings need no other evaluation and should proceed directly to repair. In some patients the diagnosis may be far less obvious, presenting without hemodynamic instability and with less specific complaints including syncope, emesis, groin, and back or flank pain. Obesity may obscure palpation of the aneurysm, making the diagnosis more difficult. In order to prevent a missed diagnosis and delay to the treatment, it is important to have a high index of suspicion for the diagnosis in any patient over the age of 50 presenting with back, abdomen, or flank pain. When the physical examination is unclear, an abdominal ultrasound can be used as a quick adjunct to the history and physical examination, especially in the emergency department. Ultrasound is sensitive in identifying aneurysms and can be used to evaluate the aorta rapidly, but is poor in determining the presence of aortic rupture. In stable patients where the diagnosis is suspected, a computed tomography (CT) scan (Fig. 1) is the most accurate method of detecting a ruptured AAA. Additionally, modern CT scanners can rapidly provide the needed anatomic information for the evaluation of patients for endovascular options. Plain abdominal radiographs (KUB) continue to be performed on the patients with abdominal pain and can be important in guiding the investigations in the emergency room. Aortic wall calcifications on the KUB confirm the presence of AAA in 75% of the patients. Moreover, loss of a psoas shadow may be apparent in the presence of rupture.
Preparation
The preoperative patient management in cases of aortic rupture should be focused and expeditious. Two large-bore intravenous cannulas, suitable for high-volume blood transfusion, should be immediately established and blood drawn for routine laboratory data and typing and immediate cross-matching for 6 units of red blood cells. Rapid transport of the patient to the operating room must be immediately undertaken. Unnecessary time can be wasted in an ill-advised attempt at resuscitation of the patient prior to transport to the operating room. If a patient is stable with a “reasonable” blood pressure, large-volume infusions and/or the use of vasopressors to raise a slightly depressed blood pressure can only serve to accelerate the rate of bleeding, or worse, result in the loss of containment of the rupture by the retroperitoneal tissues, leading to rapid decompensation and cardiac arrest. Certain best practices initially learned from the resuscitation of trauma patients (and now applied to the ruptured aneurysm patients) support the use of “permissive hypotension” to minimize ongoing hemorrhage. It is important to remember that even in stable patients the situation can change quickly and that complacency can be deadly. The surgical team is responsible for maintaining a sense of urgency and the need to move as quickly as possible to surgery. The combined efforts of anesthesia, nursing, and technical personnel can quickly prepare the patient for operative intervention and definitive management. In the operating room, a Foley catheter is inserted and a broad-spectrum antibiotic is given. Rapid transfusion devices with the capacity to warm blood products and cell washing autotransfusion devices are useful and should be prepared if available. Hypothermia is a common problem in these patients and should be ameliorated by warming the room and covering the patient with a heated air-circulating blanket. The patient is prepped and draped widely from chest to midthigh. Unstable patients should be fully prepped and draped prior to induction of anesthesia, which often leads to rapid decompensation, necessitating immediate laparotomy so that aortic clamping or compression can be accomplished as quickly as possible. Moribund patients may require laparotomy prior to induction. After surgery has commenced, useful adjuncts, such as nasogastric tubes and central venous, pulmonary artery, and radial arterial catheters, can all be placed.
The immediate goal of the surgeon is to gain proximal control of the aorta. Although some surgeons use the retroperitoneal
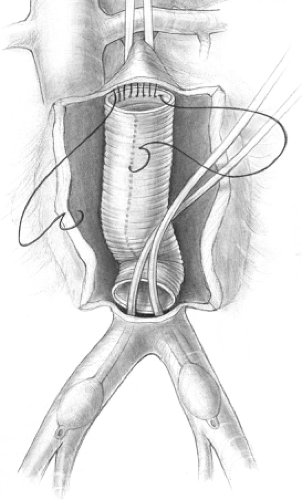
approach for ruptured aneurysm, most surgeons, including the authors, explore patients through a vertical midline incision extending from the xiphoid process to the symphysis pubis. If the patient’s hemodynamic status deteriorates rapidly when the tamponade effect of the abdominal wall is lost with laparotomy, the surgeon can compress the aorta against the spine above the celiac artery (Fig. 2) while the anesthesia team “catches up” with resuscitation. The need for continuous communication between the surgical and anesthesia teams is critical to limit severe hypotension and shock from continued rapid blood loss without adequate replacement and/or excessive volume replacement once bleeding has been controlled.
approach for ruptured aneurysm, most surgeons, including the authors, explore patients through a vertical midline incision extending from the xiphoid process to the symphysis pubis. If the patient’s hemodynamic status deteriorates rapidly when the tamponade effect of the abdominal wall is lost with laparotomy, the surgeon can compress the aorta against the spine above the celiac artery (Fig. 2) while the anesthesia team “catches up” with resuscitation. The need for continuous communication between the surgical and anesthesia teams is critical to limit severe hypotension and shock from continued rapid blood loss without adequate replacement and/or excessive volume replacement once bleeding has been controlled.
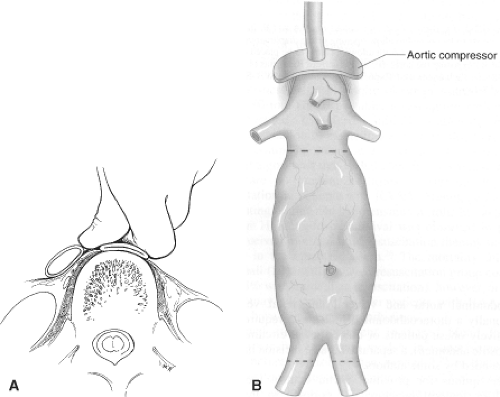 Fig. 2. A: Method of manually compressing the supraceliac aorta against the spine for temporary proximal control. Alternatively, a sponge stick, or (B) a commercially available aortic compressor can be used. (Fig. 2B: From Lindsay TF. Ruptured abdominal aortic aneurysms. In: Rutherford RB, ed. Vascular Surgery, Vol. 2, 6th ed. Philadelphia: Elsevier Saunders, 2005:1480, with permission.) |
Most ruptures occur posterior and lateral to the anterior surface of the aorta. In those cases the infrarenal neck of the aneurysm may be relatively free of hematoma and can be approached in a manner similar to that for elective aneurysm repair. The transverse colon is retracted superiorly and the small bowel eviscerated or retracted to the right side of the abdomen. The retroperitoneum is incised over the aneurysm and the duodenum mobilized to the right by incising the ligament of Treitz. Dissection commences directly over the aortic pulsation by bluntly and sharply dissecting the overlying retroperitoneal tissues, exposing the wall of the aorta. All dissection should be directed superiorly, toward the neck of the aneurysm. Extending the dissection distally into the hematoma prior to gaining proximal control should be avoided as it can lead to loss of its containment, accelerating hemorrhage. If the region of the neck of the aneurysm is obscured by hematoma, much of the dissection and exposure can be done bluntly with surgeon’s fingers avoiding sharp injury to adjacent structures. Dissection proceeds superiorly until the normal aortic wall is encountered. Usually this will be close to the point where the left renal vein crosses the anterior wall of the aorta, which should be identified to avoid inadvertent injury. The vein can be retracted superiorly slightly while the surgeon establishes a space on the lateral surfaces of the aorta with finger dissection in a longitudinal direction to permit the placement of the aortic clamp. Almost always, the segment of aorta under the left renal vein is relatively normal in infrarenal aneurysms. Occasionally, the left renal vein may need to be divided to facilitate exposure of normal aorta. The vein should be divided close to the vena cava, preserving collateral flow through the left adrenal, lumbar, and gonadal branches. Attempting to encircle the aorta is dangerous and unnecessary, running the risk of injury to underlying lumbar arterial or venous branches or the posterior wall of the aorta itself. Once the aorta has been exposed for clamping, a dose of 3,000 to 5,000 units of heparin is given intravenously. If massive hemorrhage has occurred, heparin is not given due to the likelihood of severe coagulopathy. Once the aorta is clamped, the aorta is opened longitudinally and fully exposed. With the hematoma and aneurysm sack decompressed, the iliac arteries can be more easily exposed and clamped. Care must be taken to avoid injury to the adjacent vena cava and common iliac veins. As with the aortic neck, this is best accomplished by limiting dissection to the anterior and lateral surfaces of the arteries without trying to encircle them prior to clamping. We prefer the use of soft jaw clamps. If the iliac vessels are encased in hematoma, it is best to make no attempt to expose them at all, avoiding injury to both venous structures and the ureter. Control can be achieved with a large-diameter embolectomy balloon catheter placed into the lumen of the common iliac artery from within the aorta (Fig. 3). In some cases, backbleeding from the iliac arteries is so limited that no control is necessary.
Clinical circumstances, the extent of the retroperitoneal hematoma, and the size or shape of the aneurysm may necessitate modifications to the approach. In the cases of severe hypotension or uncontrolled bleeding from intraperitoneal rupture, or whenever the surgeon feels that the extent
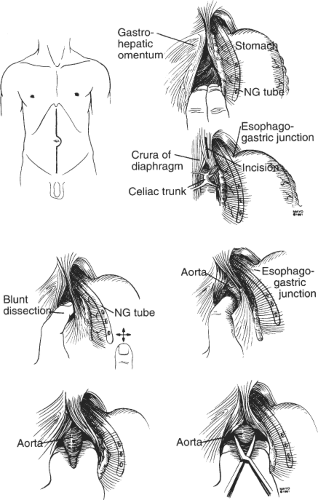
of the hematoma or the shape or size of the aneurysm makes conventional exposure too difficult or impossible, control of the supraceliac aorta can be obtained (Fig. 4). Exposure is undertaken by incising the gastrohepatic omentum to gain access to the lesser sac. The aortic pulse is palpated. The ligamentous attachments of the left lobe of the liver may need to be incised in some patients to facilitate exposure. The stomach and esophagus are displaced to the left and the crus is incised. Using both sharp and blunt finger dissection, the surgeon frees the anterior and lateral surfaces of the aorta as previously described. Many surgeons prefer to initially control the supraceliac aorta in all cases of aortic rupture. Although this does have the advantage of gaining control away from the hematoma, it can be technically difficult in obese patients and in those with previous upper abdominal surgery. Moreover, it risks injury to the esophagus, results in visceral and renal ischemia, and can cause profound hypotension from visceral reperfusion when the clamp is removed. For these reasons, the authors prefer to initially gain control of the infrarenal aorta whenever possible, reserving supraceliac control for the selected circumstances previously described. Should supraceliac control be necessary, clamp time should be as brief as possible to minimize ischemic injury to the liver, bowel, and kidneys as this may contribute to the development of multisystem organ failure postoperatively.
of the hematoma or the shape or size of the aneurysm makes conventional exposure too difficult or impossible, control of the supraceliac aorta can be obtained (Fig. 4). Exposure is undertaken by incising the gastrohepatic omentum to gain access to the lesser sac. The aortic pulse is palpated. The ligamentous attachments of the left lobe of the liver may need to be incised in some patients to facilitate exposure. The stomach and esophagus are displaced to the left and the crus is incised. Using both sharp and blunt finger dissection, the surgeon frees the anterior and lateral surfaces of the aorta as previously described. Many surgeons prefer to initially control the supraceliac aorta in all cases of aortic rupture. Although this does have the advantage of gaining control away from the hematoma, it can be technically difficult in obese patients and in those with previous upper abdominal surgery. Moreover, it risks injury to the esophagus, results in visceral and renal ischemia, and can cause profound hypotension from visceral reperfusion when the clamp is removed. For these reasons, the authors prefer to initially gain control of the infrarenal aorta whenever possible, reserving supraceliac control for the selected circumstances previously described. Should supraceliac control be necessary, clamp time should be as brief as possible to minimize ischemic injury to the liver, bowel, and kidneys as this may contribute to the development of multisystem organ failure postoperatively.
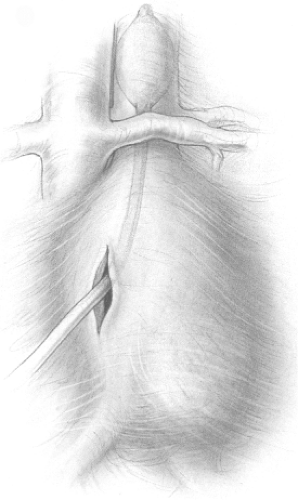
Occasionally, when opening the abdomen, a free intraperitoneal rupture may be encountered with a massive hematoma. Manual compression at the diaphragm should be immediately undertaken and maintained by an assistant. Temporary control of hemorrhage may be accomplished by inserting a thumb into the aortic tear if it is encountered. The surgeon has no choice other than to enter the hematoma directly to both gain stable proximal control and expose the neck for the proximal anastomosis. It may be necessary for the proximal anastomosis to be completed with aortic control maintained by compression at the diaphragm by the assistant. Once the anastomosis is completed, the graft is clamped just distal to the anastomosis. Alternatively, a Foley catheter with a 30-mL balloon can be inflated in the suprarenal aorta once it is opened or through the aortic tear (Fig. 5). Dissection in the hematoma under these circumstances is especially hazardous and should be done carefully to avoid injury to the adjacent structures. Generally, the hematoma will have facilitated the dissection
by separating the duodenum and retroperitoneal tissues away from the aorta. On rare occasions gaining proximal control at any level in the abdomen may be so difficult that left thoracotomy and aortic cross-clamping just above the diaphragm may be the only solution.
by separating the duodenum and retroperitoneal tissues away from the aorta. On rare occasions gaining proximal control at any level in the abdomen may be so difficult that left thoracotomy and aortic cross-clamping just above the diaphragm may be the only solution.
Aneurysm Repair
Once proximal and distal control has been established, the anesthesia team continues the resuscitation while the surgical team commences with the repair. Resuscitation can be aided by the use of pulmonary arterial catheters or transesophageal echocardiography. Both are routinely used in our patients. Reducing hypothermia and adequate replacement of platelets and clotting factors are critical at this juncture to reduce or correct coagulopathy. It is important that the surgical team communicate regularly with the anesthesia team, alerting them to ongoing blood loss and warning them well in advance of restoration of blood flow to the extremities, which can cause severe hypotension, especially in the presence of continued hypovolemia and metabolic acidosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree