Robotic Pancreaticoduodenectomy
A. James Moser
Herbert J. Zeh III
Introduction
The major technical aspects of surgical resection for periampullary tumors were established in the early 20th century and have not changed significantly for nearly 100 years. Walter Kausch pioneered surgical resection of the pancreatic head for ampullary carcinoma in 1909. The procedure underwent two major technical revisions: the first in 1935 and the second in 1978. Allen O. Whipple devised a single-stage operation to resect the pancreatic head and published the first case series of successful pancreaticoduodenectomy (PD) in 1945. This report described (1) a single-stage procedure for resection and reconstruction of the pancreatic head, (2) avoidance of cholecystoenterostomy by implantation of the bile duct into the jejunum, and (3) anastomosis of the pancreatic duct with the jejunum. The modern form of pylorus-preserving PD was described by Traverso and Longmire in 1978 in an attempt to reduce the severity of postgastrectomy syndrome.
Postoperative mortality rates as high as 30% prevented widespread use of PD for several decades. Attention to surgical detail combined with advances in critical care and anesthesia led to significant improvements in postoperative outcome. In 1968, John Howard reported a consecutive series of 41 pancreaticoduodenectomies without a death. John Cameron and colleagues at Johns Hopkins standardized the technical aspects of PD and established the benchmark for modern outcomes with recent large series reporting a morbidity rate between 30% and 40% with a mortality rate of 1% to 3%.
Recent refinements of PD have focused on minimally invasive adaptations. Gagner and Pomp performed the first laparoscopic PD in 1994, a procedure which lasted nearly 24 hours. Subsequent series by Kendrick and Cusati, and Palanivelu and colleagues demonstrate in highly selected patients that minimally invasive PD can be performed with outcomes comparable to large series of open PD. Although advanced laparoscopic surgery is widely performed, advanced procedures that require complicated reconstruction such as PD remain limited to a few specialized centers. Recently, Palanivelu and colleagues presented 75 cases, and Kendrick and Cusati reported 62 cases of totally laparoscopic PDs. Nevertheless, fewer than 200 reports of laparoscopic PDs are found in the English literature since Gagner’s description in 1994. The slow implementation of laparoscopic PD is an acknowledgement of the inherent limitations of current instrumentation, such as limited range of instrument motion, reduced fine motor control, poor ergonomics, and two-dimensional vision.
Technique for Robotic-Assisted Pancreaticoduodenectomy
Robotic assistance has the potential to overcome many shortcomings of traditional laparoscopy by providing magnified stereoscopic visualization, 540-degree movement of the surgical instruments, and improved surgical precision by eliminating the surgeon’s tremor. These technological innovations allow complex resections and anastomotic reconstructions to be performed with techniques comparable to open surgery.
The authors present their selection criteria for robotic-assisted pancreaticoduodenectomy (RAPD), technical description, and preliminary outcomes. This approach duplicates the traditional open surgical techniques using minimal access techniques. Variations of this surgical procedure and its preliminary outcomes have been published by groups led by Giulianotti, Melvin, and Moser and Zeh.
Selection Criteria
Safety and transparency of surgical outcomes are the primary mission of an advanced procedure in its early stages of adoption. All potential candidates for robotic pancreaticoduodenectomy participate in an IRB-approved robotic surgery registry. All robotic pancreaticoduodenectomies are performed under the direction of an expert pancreatic surgeon familiar with open PD and capable of carrying out venous resection and reconstruction whenever indicated. Surgical trainees are incorporated into the surgical team in a stepwise fashion as their training and expertise with Robotic Technology permit.
Patients with periampullary malignancies, who are potential candidates for robotic pancreaticoduodenectomy, undergo individualized treatment planning based on a validated predictive model to maximize the likelihood of achieving an R0 surgical resection. The purpose of risk stratification is to minimize the potential for robotic surgery to compromise oncologic principles among patients at highest risk. The model stratifies patients into low risk and high risk for non-R0 surgical outcomes based on the findings of preoperative computed tomography (CT) and endoscopic ultrasonography (EUS). We do not currently offer the robotic approach to high-risk patients but instead advise traditional open PD if they choose not to participate in a clinical trial of neoadjuvant therapy. Low-risk patients are offered robotic pancreaticoduodenectomy after a detailed consent process and enrollment in a prospective registry.
Validated predictors of oncologic outcome include: (1) any evidence of arterial or venous vascular involvement on CT; (2) the imputed preoperative stage by combining EUS T-stage and N-stage data according to the criteria of the American Joint Committee on Cancer (6th edition), and (3) largest EUS tumor dimension greater than 2.6 cm. Evidence for vascular involvement by CT scan includes minimal abutment of the superior mesenteric or hepatic arteries without extension to the celiac axis, as well as any preoperative suspicion that tumor involves the superior mesenteric vein (SMV)-portal vein (PV) confluence. The prediction rule (Fig. 1) classifies operative findings of metastatic or locally advanced disease as well as positive resection margins as treatment failures.
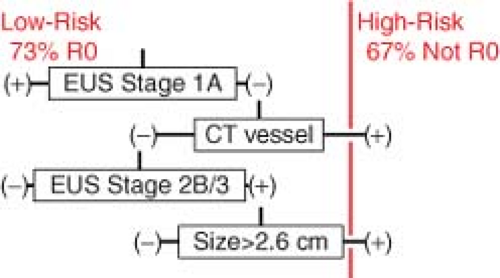 Fig. 1. Validated prediction rule based on imaging factors identified during preoperative pancreas mass protocol computed tomography and endoscopic ultrasonography.15 |
A patient is considered a good candidate for R0 resection (low risk) and should undergo surgery as primary therapy if: (a) the EUS stage is 1A; (b) there is no vascular
involvement, and the EUS stage is greater than 1A but less than 3; or (c) there is no vascular involvement and EUS stage 2B, but the largest tumor dimension is <2.6 cm. Otherwise, a patient is classified at high risk for resection. Using these predictors, the overall resection rate among low-risk patients was significantly greater (92%) than that of the high-risk group (53%; P < 0.001). Low-risk patients achieved R0 status more frequently than high-risk patients (73% vs. 33% R0, P < 0.001), despite resection and reconstruction of the PV whenever indicated in both groups. Additional operative findings distinguishing the two risk groups included a greater proportion of unresectable, locally advanced tumors (17% vs. 0%, P < 0.007) as well as unexpected metastatic disease (30% vs. 8%, P < 0.026) in the high-risk group. High predicted risk of surgical failure corresponded to more advanced stages of disease on final surgical pathology, and also correlated with shorter postoperative overall survival. Median survival of low-risk patients was 20.3 months, compared with 12.1 months in those considered at high risk (P < 0.02).
involvement, and the EUS stage is greater than 1A but less than 3; or (c) there is no vascular involvement and EUS stage 2B, but the largest tumor dimension is <2.6 cm. Otherwise, a patient is classified at high risk for resection. Using these predictors, the overall resection rate among low-risk patients was significantly greater (92%) than that of the high-risk group (53%; P < 0.001). Low-risk patients achieved R0 status more frequently than high-risk patients (73% vs. 33% R0, P < 0.001), despite resection and reconstruction of the PV whenever indicated in both groups. Additional operative findings distinguishing the two risk groups included a greater proportion of unresectable, locally advanced tumors (17% vs. 0%, P < 0.007) as well as unexpected metastatic disease (30% vs. 8%, P < 0.026) in the high-risk group. High predicted risk of surgical failure corresponded to more advanced stages of disease on final surgical pathology, and also correlated with shorter postoperative overall survival. Median survival of low-risk patients was 20.3 months, compared with 12.1 months in those considered at high risk (P < 0.02).
Robotic-assisted minimally invasive resection of the pancreatic head duplicates published methods for open PD. Incorporation of this technology into the pancreaticoduodenectomy was greatly facilitated by the initial involvement of expert pancreatic surgeons having a combined experience in excess of 1,000 open pancreaticoduodenectomies. This teamwork and diversity in operative experience allowed for rapid evolution of the approach. Like open surgery, the technique requires four-handed cooperation to retract and expose critical structures, the anatomy of which may be distorted by tumor, body habitus, and pancreatitis. Exposure and safe control of bleeding from major vascular requires strong familiarity with the anatomy, skilled collaboration, and the mutual ability to anticipate each other’s movements.
Patient Position
The endotracheal tube, nasogastric drain, EKG monitoring equipment, and central venous catheters must be functional and securely positioned before the operation commences because access to the head of the table becomes severely restricted during the robotic portion of the operation. The patient is positioned supine on a split-leg table with the right arm tucked and the left arm extended. The distance between the umbilicus and the head of the table is measured to keep the robotic camera arm within design parameters (the “sweet spot”). An upper body convective warming blanket is used to maintain normothermia.
Port Position
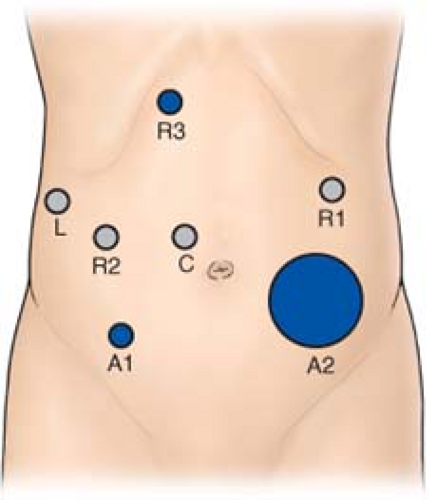
Seven laparoscopic ports are required (Fig. 2). The 5-mm optical separator is used to access the peritoneal cavity in the left subcostal region. The camera port is placed 2 to 3 cm to the right of the midline at the level of the umbilicus to improve exposure of the PV. Two 5-mm ports (R1 and R3) are placed approximately in the right upper quadrant and later converted to 8-mm robotic trocars. A 5-mm port for the laparoscopic liver retractor is inserted in the anterior axillary line. Two assistant ports (A1 and A2) are placed in the lower quadrants. Once resectability is ensured, a 5-cm extraction incision is created in the left lower quadrant and a GelPoint® (Applied Medical, Rancho Santa Margarita, CA, USA) access device is placed through which a 10-mm port is inserted to permit the passage of needles, staplers, and extraction bags as required.
Instruments
Laparoscopic instruments are used to explore the abdomen, mobilize the right colon, elevate the pancreatic head from the retroperitoneum (Kocher maneuver), and divide the proximal duodenum and jejunum. Free mobility of the table during laparoscopy permits gravity to act as a retractor of the hollow viscera. The dissection begins with a 45-degree-angled laparoscope, atraumatic graspers, suction, and the LigaSure® (Covidien, Boulder, CO, USA). After mobilization of the pancreatic head and division of the duodenum, the daVinci Si robotic platform (Intuitive Surgical, Sunnyvale, CA, USA) is used for the portal dissection and subsequent reconstruction, assisted by the laparoscopic cosurgeon seated between the patient’s legs.
Step 1: Mobilization of the Right Colon and Pancreatic Head
Following insufflation and laparoscopic staging to exclude unrecognized metastases (Fig. 3), a suture is used to anchor the falciform ligament to the anterior abdominal wall in order to elevate the left lateral segment of the liver. The retroperitoneal attachments of the hepatic flexure are divided, and the right colon is rotated medially down to the terminal ileum to expose the SMV at the root of the small-bowel mesentery. This action is performed from the left side of the table with the LigaSure device and an atraumatic grasper (ports R1 and A2). A flexible liver retractor is inserted through a 5-mm port in the right anterior axillary line to retract the gallbladder and expose the porta hepatis. The retroperitoneal investments of the third portion of the duodenum are divided, and the pancreatic head is elevated from the retroperitoneum up to the origin of the superior mesenteric artery (SMA).
Next, an extended Kocher maneuver is performed from the right side of the table to release the proximal jejunum from the ligament of Treitz. The jejunum is pulled into the right upper quadrant and is transected with a 3.5-mm linear cutting stapler <10 cm distal to the former ligament of Treitz. The jejunum is marked with an Endostitch (Covidien, Boulder, CO, USA) 50 to 60 cm downstream to mark the intended location of the future duodenojejunostomy.
Step 2: Division of the Gastrocolic Omentum, Proximal Duodenum, and Jejunum
The gastrocolic omentum is divided with the LigaSure to expose the posterior surface of the stomach (Fig. 4) and the right gastroepiploic vascular
pedicle. The groove between the gastroepiploic vascular pedicle and the duodenum is opened with the LigaSure, elevating the first portion of the duodenum from the pancreatic head. The right gastric artery is identified, mobilized from the hepatic artery, and clipped and divided to complete the mobilization of the duodenum. The duodenum is divided with a linear cutting stapler (port A1). The gastroepiploic pedicle is divided with a vascular stapler, preserving the vessels along the greater curve but leaving the prepyloric lymph nodes in continuity with the specimen. The jejunum is pulled to the left beneath the mesenteric vessels and the Endostitch used to locate the intended site of the duodenojejunostomy. The jejunum is then sewn to the greater curvature of the stomach to allow for easy identification during reconstruction.
pedicle. The groove between the gastroepiploic vascular pedicle and the duodenum is opened with the LigaSure, elevating the first portion of the duodenum from the pancreatic head. The right gastric artery is identified, mobilized from the hepatic artery, and clipped and divided to complete the mobilization of the duodenum. The duodenum is divided with a linear cutting stapler (port A1). The gastroepiploic pedicle is divided with a vascular stapler, preserving the vessels along the greater curve but leaving the prepyloric lymph nodes in continuity with the specimen. The jejunum is pulled to the left beneath the mesenteric vessels and the Endostitch used to locate the intended site of the duodenojejunostomy. The jejunum is then sewn to the greater curvature of the stomach to allow for easy identification during reconstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree