Table 26-1. Disease-Modifying Antirheumatic Drugs
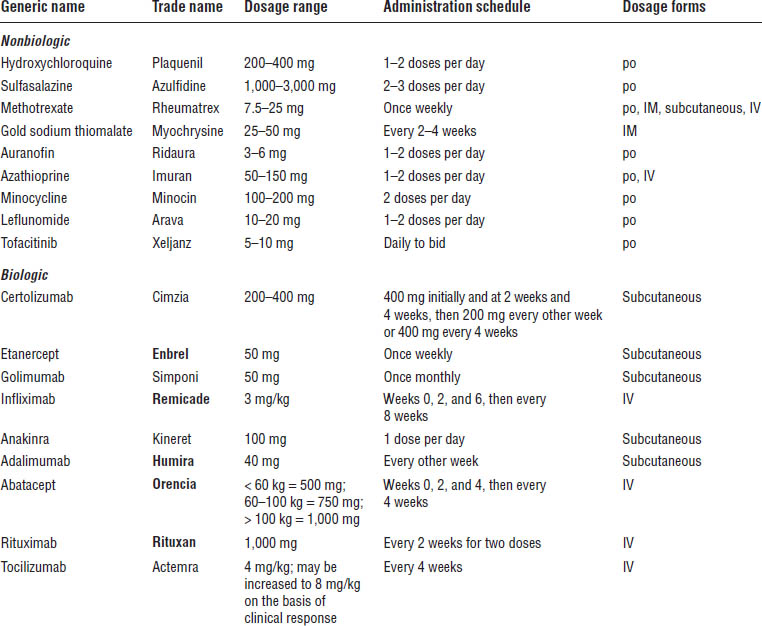
IM, intramuscular; IV, intravenous.
Boldface indicates one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
NSAIDs
Mechanism of action
NSAIDs prevent prostaglandin formation by inhibiting the action of the enzyme cyclooxygenase. The antithrombotic effect of aspirin occurs by an irreversible inhibition of platelet cyclooxygenase. This irreversible inhibition is unique to aspirin, because the remaining NSAIDs do so in a reversible manner.
Patient instructions
NSAIDs should be taken with food or milk to decrease gastrointestinal (GI) intolerance. Patients should report any dark or black stools, abdominal pain, or swelling to their health care provider immediately. Studies indicate that the optimal times for taking an NSAID might be after the evening meal and immediately on awakening. Patients with a hypersensitivity to aspirin should not take NSAIDs.
Figure 26-1. 2012 American College of Rheumatology Recommendations Update for the Treatment of Early Rheumatoid Arthritis (RA), Defined as a Disease Duration < 6 Months
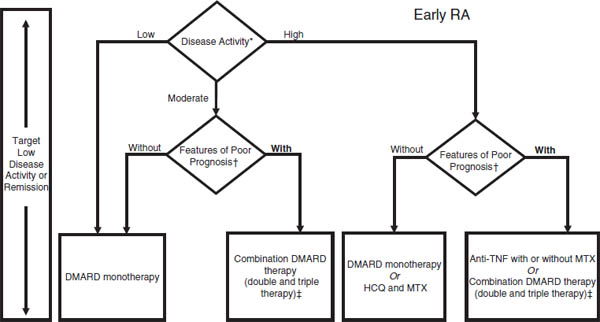
For the level of evidence supporting each recommendation, please see Supplementary Appendix 7 (available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658). DMARD = disease-modifying antirheumatic drug (includes hydroxychloroquine [HCQ], leflunomide [LEF], methotrexate [MTX], minocycline, and sulfasalazine); anti-TNF = anti–tumor necrosis factor.
* Definitions of disease activity are discussed in Tables 2 and 3 and Supplementary Appendix 4 (available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658) and were categorized as low, moderate, or high.
† Patients were categorized based on the presence or absence of 1 or more of the following poor prognostic features: functional limitation (e.g., Health Assessment Questionnaire score or similar valid tools), extraarticular disease (e.g., presence of rheumatoid nodules, RA vasculitis, Felty’s syndrome), positive rheumatoid factor or anti–cyclic citrullinated peptide antibodies (33–37), and bony erosions by radiograph (38).
‡ Combination DMARD therapy with 2 DMARDs, which is most commonly MTX based, with some exceptions (e.g., MTX + HCQ, MTX + LEF, MTX + sulfasalazine, and sulfasalazine + HCQ), and triple therapy (MTX + HCQ + sulfasalazine) as defined in Table 2.
Reprinted with permission from the American College of Rheumatology. Singh JA, et al., 2012.
Adverse drug events
Compared with patients with osteoarthritis, patients with RA on NSAID therapy are at increased risk for a serious complication.
As with aspirin, NSAIDs cause platelet dysfunction. Unlike aspirin, however, this effect is readily reversible with discontinuation of the medication.
All NSAIDs are capable of causing GI intolerance and peptic ulceration. Risk factors for the development of peptic ulcer disease include advanced age, history of previous ulcer, concomitant use of corticosteroids or anticoagulants, higher dosage of NSAID, use of multiple NSAIDs, or serious underlying disease. Options to decrease the risk of developing GI ulceration include using a selective COX-2 inhibitor or adding a proton pump inhibitor to the patient’s regimen. Misoprostol, an oral prostaglandin analog, may be added at a dose of 100–200 mcg four times daily to prevent ulceration but is not as well tolerated because of diarrhea. Misoprostol is available in combination with diclofenac and sold under the trade name Arthrotec. A 2008 joint consensus statement by the American College of Cardiology Foundation, the American Heart Association, and the American College of Gastroenterology recommends that patients with a history of ulcer disease or with risk factors for ulceration be treated with a proton pump inhibitor while on NSAID therapy. If the patient is taking low-dose aspirin for cardiovascular protection, a nonselective NSAID may be used in combination. However, ibuprofen should be avoided or given 2 hours later than the aspirin dose because it can render the aspirin less effective. COX-2 inhibitors should not be used in patients with cardiovascular disease.
Figure 26-2. 2012 American College of Rheumatology (ACR) Recommendations Update for the Treatment of Established Rheumatoid Arthritis (RA), Defined as a Disease Duration ≥ 6 Months or Meeting the 1987 ACR Classification Criteria
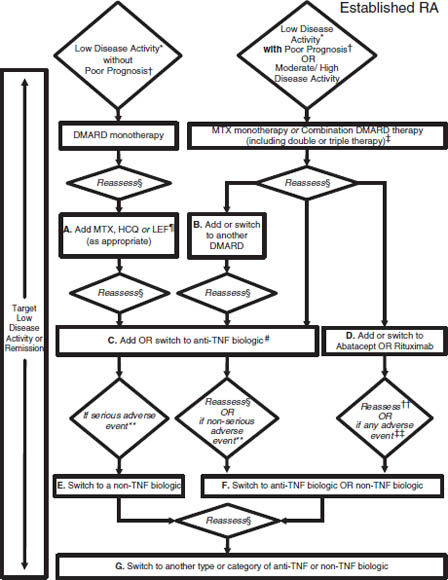
Depending on a patient’s current medication regimen, the management algorithm may begin at an appropriate rectangle in the figure, rather than only at the top of the figure. Disease-modifying antirheumatic drugs (DMARDs) include hydroxychloroquine (HCQ), leflunomide (LEF), methotrexate (MTX), minocycline, and sulfasalazine (therapies are listed alphabetically; azathioprine and cyclosporine were considered but not included). DMARD monotherapy refers to treatment in most instances with HCQ, LEF, MTX, or sulfasalazine; in few instances, where appropriate, minocycline may also be used. Anti–tumor necrosis factor (anti-TNF) biologics include adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab. Non-TNF biologics include abatacept, rituximab, or tocilizumab (therapies are listed alphabetically). For the level of evidence supporting each recommendation, please see Supplementary Appendix 7 (available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658).
* Definitions of disease activity are discussed in Tables 2 and 3 and Supplementary Appendix 4 (available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658) and were categorized as low, moderate, or high.
† Features of poor prognosis included the presence of 1 or more of the following: functional limitation (e.g., Health Assessment Questionnaire score or similar valid tools), extraarticular disease (e.g., presence of rheumatoid nodules, RA vasculitis, Felty’s syndrome), positive rheumatoid factor or anti–cyclic citrullinated peptide antibodies (33–37), and bony erosions by radiograph (38).
‡ Combination DMARD therapy with 2 DMARDs, which is most commonly MTX based, with few exceptions (e.g., MTX + HCQ, MTX + LEF, MTX + sulfasalazine, sulfasalazine + HCQ), and triple therapy (MTX + HCQ + sulfasalazine).
§ Reassess after 3 months and proceed with escalating therapy if moderate or high disease activity in all instances except after treatment with a non-TNF biologic (rectangle D), where reassessment is recommended at 6 months due to a longer anticipated time for peak effect.
¶ LEF can be added in patients with low disease activity after 3–6 months of minocycline, HCQ, MTX, or sulfasalazine.
# If after 3 months of intensified DMARD combination therapy or after a second DMARD has failed, the option is to add or switch to an anti-TNF biologic.
** Serious adverse events were defined per the U.S. Food and Drug Administration (FDA; see below); all other adverse events were considered nonserious adverse events.
†† Reassessment after treatment with a non-TNF biologic is recommended at 6 months due to anticipation that a longer time to peak effect is needed for non-TNF compared to anti-TNF biologics.
‡‡ Any adverse event was defined as per the U.S. FDA as any undesirable experience associated with the use of a medical product in a patient. The FDA definition of serious adverse event includes death, life-threatening event, initial or prolonged hospitalization, disability, congenital anomaly, or an adverse event requiring intervention to prevent permanent impairment or damage.
Reprinted with permission from the American College of Rheumatology. Singh JA, et al., 2012.
Table 26-2. Drug Therapy with Nonsteroidal Anti-Inflammatory Drugs
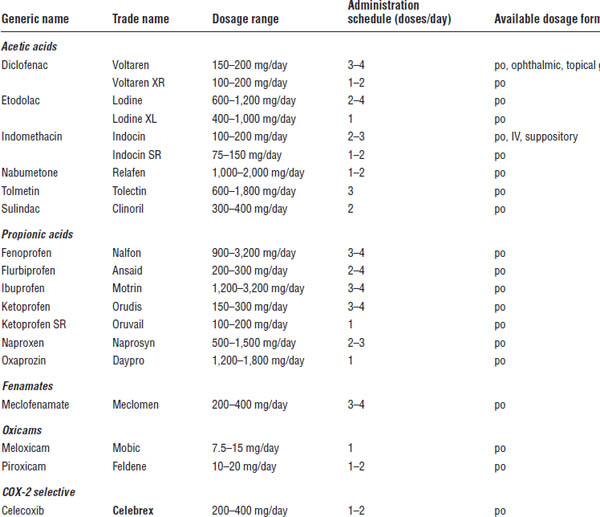
IV, intravenous.
Hepatic failure has been reported with NSAID use.
Renal blood flow can be decreased by NSAIDs, which may lead to permanent renal damage. Prostaglandins are responsible for maintaining the patency of the afferent renal tubule. Inhibition by NSAIDs decreases glomerular filtration pressure, resulting in decreased blood flow. Because of this mechanism, patients with hypertension, severe vascular disease, and kidney or liver problems and those taking diuretics must be monitored closely.
Central nervous system (CNS) side effects such as dizziness, drowsiness, and confusion may occur with all NSAIDs.
Within the class, some drug-specific adverse reactions occur. Meclofenamate, for example, has a high incidence (> 10%) of abdominal cramping and diarrhea. Paradoxically, indomethacin tends to have more severe CNS adverse effects, such as headache.
Concern exists about NSAIDs and their risk of cardiovascular events. The U.S. Food and Drug Administration (FDA) now requires that manufacturers include a black box warning regarding the potentially serious cardiovascular and GI adverse events associated with these drugs. NSAIDs should not be used in heart failure.
Drug–drug interactions
Interactions are the same as those associated with aspirin. Ibuprofen may diminish the antiplatelet mechanism of aspirin if it is taken before aspirin or taken daily on a scheduled basis. It is recommended that aspirin be taken 2 hours before taking ibuprofen.
COX-2 inhibitors
COX-1 is the isoenzyme constitutively found in most tissues that produce the prostaglandins PGI2 and PGE2, which protect the gastric barrier, and thromboxane A2, which is responsible for platelet function. COX-2 is the inducible isoenzyme present at sites of inflammation. COX-2 is also found in the brain, the kidneys, and the reproductive organs. Celecoxib (Celebrex) has been shown to have lower incidence of endoscopically demonstrated gastroduodenal lesions than do ibuprofen, naproxen, and diclofenac. The lower risk for GI complications is apparently eliminated when patients take low-dose aspirin concomitantly.
Celecoxib
Mechanism of action
Celecoxib selectively inhibits prostaglandin synthesis by specifically targeting the COX-2 isoenzyme.
Patient instructions
Patients with a history of allergic reaction to sulfonamides should avoid the use of celecoxib.
Adverse drug events
Although the rates of GI ulceration have been demonstrated to be lower with COX-2 inhibitors than with traditional NSAIDs, the risk is not completely eliminated. In addition, the risk of dyspepsia, abdominal pain, and nausea is not significantly less with COX-2 inhibitors than with traditional NSAIDs. Celecoxib now contains a black box warning regarding cardiovascular and GI risk associated with its use (as described later).
Drug–drug interactions
Interactions are the same as those associated with aspirin.
Parameters to monitor
Complete blood count (CBC) as well as creatinine should be monitored at least yearly.
Other aspects
The FDA recommended the voluntary removal of valdecoxib (Bextra) from the market in 2005 because of the lack of adequate data on the cardiovascular safety of its long-term use and the recent data demonstrating increased cardiovascular risk in short-term coronary artery bypass graft (CABG) patients. This risk is in addition to that of potentially life-threatening skin reactions. Merck removed rofecoxib (Vioxx) from the market in September 2004 because its use was shown to be associated with an increased cardiovascular risk in the VIGOR (Vioxx Gastrointestinal Outcomes Research), APPROVE (Adenomatous Polyp Prevention on Vioxx), and VICTOR (Vioxx in Colorectal Therapy, Definition of Optimal Regimen) trials. The FDA has concluded that the benefits of celecoxib outweigh the risks in properly selected and informed patients. Celecoxib contains a black box warning about cardiovascular and GI risk. Patients with a high risk of cardiovascular events should not use celecoxib, including CABG patients. Low doses of celecoxib (200 mg per day) do not seem to be associated with increased risk.
Disease-modifying antirheumatic drugs
Unlike the NSAIDs, DMARDs have the ability to reduce or prevent joint damage and preserve joint integrity and function. The ACR recommends that patients with an established diagnosis of RA be offered treatment with DMARDs. Biologic DMARDs are reserved for use after failure of nonbiologic agents, unless the patient has early disease with high activity and poor prognosis risk factors. Methotrexate is typically selected for initial therapy because of its track record to induce long-term response. Methotrexate or leflunomide may be used as monotherapy in patients with all disease durations and activity regardless of poor prognostic features. Unfortunately, all DMARDs tend to lose effectiveness over time.
Nonbiologic DMARDs
Hydroxychloroquine
Mechanism of action
Hydroxychloroquine (Plaquenil) may inhibit interleukin-1 release by monocytes, thereby decreasing macrophage chemotaxis and phagocytosis. It also inhibits the function of toll-like receptors that contribute to autoimmune disease, limiting B cell and dendritic cell activation.
Patient instructions
Beneficial effect may not be seen until 1–6 months of use. Patients should report any changes in vision to their health care provider immediately.
Adverse drug events
The most serious potential adverse effect associated with hydroxychloroquine is retinal damage that can lead to vision loss. This damage is caused by the deposition of the drug in the melanin layer of the cones. A cumulative dose of 800 g and age > 70 years increase the risk. Hydroxychloroquine may also cause rash, abdominal cramping, diarrhea, myopathy, skin pigment changes, and peripheral neuropathy.
Parameters to monitor
Ophthalmic evaluations should be performed at baseline. If the patient has no risk factors (liver disease, retinal disease, age > 60) and the baseline examination is normal, the American College of Ophthalmology recommends no further testing for 5 years. High-risk patients should have annual exams.
Dose
The dose is 6–7.5 mg/kg of lean body weight daily or 200 mg bid (maximum dose).
Sulfasalazine
Mechanism of action
The intestinal flora breaks sulfasalazine (Azulfidine) down to 5-aminosalicylic acid and sulfapyridine, the active moiety in RA. Sulfapyridine likely inhibits endothelial cell proliferation, reactive oxygen species, and cytokines. In addition, it has been shown to slow radiographic progression of RA.
Patient instructions
Sulfasalazine may produce effects more quickly (within 1 month) than hydroxychloroquine. A coated tablet form may help reduce adverse GI effects.
Adverse drug events
The most common adverse reactions associated with sulfasalazine include headache, GI intolerance, dysgeusia, rash, leukopenia, and thrombocytopenia. A reversible oligozoospermia can occur in up to 33% of males and thus may impair fertility.
Drug–drug interactions
Sulfasalazine may inhibit the absorption of folic acid.
Parameters to monitor
Patient tests should include baseline CBC and liver function tests (LFTs). Patients should then have a CBC every 2–4 weeks for the first 3 months and then once every 3 months thereafter. Patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency should not receive sulfasalazine.
Dose
Begin with 500 mg daily, titrated up to 1–3 g/day divided bid.
Other aspects
Patients should receive a pneumococcal vaccination before initiation.
Methotrexate
Mechanism of action
Methotrexate (Rheumatrex) inhibits dihydrofolate reductase, which reduces dihydrofolate to tetrahydrofolate. Tetrahydrofolate can be used as a carrier of single carbon units for the synthesis of nucleotides and thymidylate. Therefore, methotrexate interferes with deoxyribonucleic acid (DNA) synthesis, repair, and cellular replication.
Patient instructions
Patients should not take this medicine more than once per week. Daily dosing would be disastrous. Patients should be instructed not to drink any alcohol. Methotrexate is a teratogen and is pregnancy category X. Pregnancy and lactation should be avoided. Females should wait 3–6 months after discontinuation before conception, and males should wait 3 months before fathering a child. Doses up to 30 mg weekly do not affect female fertility but can cause a reversible male sterility.
Adverse drug events
■ Liver: Methotrexate may cause liver damage. People with diabetes, liver problems, obesity, and psoriasis and those who are elderly or alcoholic are at higher risk. If LFTs are more than three times the upper limit of normal, methotrexate should be discontinued.
■ Bone marrow: Leukopenia, thrombocytopenia, and pancytopenia are rare but serious adverse events associated with methotrexate therapy.
■ Lung: Pulmonary toxicity is thought to occur in 0.1–1.2% of people who take methotrexate. Risk factors for the development of pulmonary toxicity include age; diabetes; rheumatoid involvement of the lungs; protein in the urine; and previous use of sulfasalazine, oral gold, or penicillamine.
■ GI: Nausea, vomiting, and stomatitis occur with an incidence of 5–30%.
Drug–drug interactions
Aspirin and other NSAIDs may increase methotrexate concentrations by as much as 30–35%. Trimethoprim-sulfamethoxazole may cause additive hematologic abnormalities because of its similar affinity for dihydrofolate reductase.
Parameters to monitor
CBC, LFTs, albumin, and creatinine should be monitored every 2–4 weeks for the first 3 months and every 8–12 weeks thereafter. Patients at risk for hepatitis B and C should be screened prior to initiation.
Other aspects
Taking folate supplements may help minimize adverse effects such as liver toxicity and should be regularly prescribed with methotrexate. Folic acid in doses up to 3 mg/day has proven effective and does not diminish methotrexate activity.
Dose
The dose is 7.5–25 mg once weekly.
Leflunomide
Mechanism of action
Leflunomide (Arava) inhibits dihydroorotate dehydrogenase (an enzyme involved in de novo pyrimidine synthesis) and has antiproliferative activity. Several in vivo and in vitro experimental models have demonstrated its anti-inflammatory effect.
Patient instructions
Leflunomide is pregnancy category X. Women taking leflunomide who wish to become pregnant should follow the drug elimination procedure outlined below under “Other aspects.” Patients on leflunomide should be instructed not to drink any alcohol.
Adverse drug events
Diarrhea, elevated LFTs, alopecia, hypertension, and rash have been reported with leflunomide therapy.
Drug–drug interactions
An increased risk of liver toxicity exists when leflunomide is used in conjunction with methotrexate. Rifampin causes a 40% increase in levels of leflunomide’s active metabolite, M1.
Parameters to monitor
CBC, LFTs, albumin, and creatinine should be monitored every 2–4 weeks for the first 3 months and every 8–12 weeks thereafter. If alanine aminotransferase exceeds two times the upper limit of normal, reduce the dose of leflunomide to 10 mg/day. Patients at risk for hepatitis B and C should be screened prior to initiation.
Kinetics
After absorption, 80% of the parent compound is converted to the active metabolite, M1, which is responsible for all of leflunomide’s activity. Because the half-life is 2 weeks, a loading dose is necessary. In addition, M1 undergoes extensive enterohepatic recirculation.
Other aspects
Begin the following drug elimination procedure if a patient decides to become pregnant: 8 g of cholestyramine three times daily for 11 days; plasma levels of M1 < 0.02 mg/L must be verified on two separate occasions at least 14 days apart.
Many randomized controlled trials have established leflunomide as an alternative to methotrexate as monotherapy.
Dose
The dose is 100 mg daily for 3 days (loading dose), then 20 mg daily.
Gold compounds
The intramuscular (IM) gold compounds are gold sodium thiomalate (Myochrysine) and aurothioglucose (Solganal). Auranofin (Ridaura) is given orally.
Mechanism of action
The mechanism of action of gold compounds is currently unknown; they appear to suppress the synovitis seen in RA. Current research indicates that they may stimulate specific protective factors, such as interleukin-6 and interleukin-10.
Patient instructions
Patients receiving gold therapy should avoid prolonged sun exposure, which may increase the risk of serious rash.
Adverse drug events
IM gold
Patients may experience an immediate “nitroid reaction” (i.e., flushing, weakness, dizziness, sweating, syncope, hypotension). Rash is the single-largest adverse effect associated with gold therapy. The rash may range from simple erythema to exfoliative dermatitis. Gold therapy may also cause proteinuria or microscopic hematuria. Rarely, immunologic glomerulonephritis may occur, in which case gold therapy should be permanently discontinued. Leukopenia and thrombocytopenia occur with a 1–3% incidence.
Oral gold
Adverse reactions are similar to those associated with the IM formulation. However, GI complaints of nausea, diarrhea, emesis, and dysgeusia are higher.
Drug–drug interactions
Patients receiving concomitant penicillamine therapy may be subject to an increased risk of toxicity associated with gold therapy. The risk of rash is higher when gold therapy is used with hydroxychloroquine.
Parameters to monitor
At baseline, all patients should have a CBC, platelet count, creatinine profile, and urinalysis for protein. For patients receiving IM therapy, a CBC, platelet count, and urinalysis are recommended every 1–2 weeks for the first 20 weeks and then again at the time of each (or every other) injection. Those on oral therapy should have a CBC, platelet count, and urinalysis for protein every 4–12 weeks.
Other aspects
Aurothioglucose may have a lower rate of injection reactions; its sesame seed formulation slows absorption.
Dose
IM gold
A 10 mg test dose IM is followed by a 25 mg test dose on week 2 and then weekly 50 mg doses until a cumulative dose of 1 g is achieved. The maintenance regimen is 50 mg every 2 weeks for 3 months or until 1.5 g is given; then every 3 weeks; and then monthly.
Oral gold
The dose is 3 mg bid up to 3 mg tid.
Biologic DMARDs
Anti–tumor necrosis factor therapy
The drugs used in anti–tumor necrosis factor (anti-TNF) therapy are infliximab (Remicade), etanercept (Enbrel), adalimumab (Humira), golimumab (Simponi), and certolizumab (Cimzia).
Mechanism of action
Composed of human constant and murine variable regions, infliximab is an antibody that binds specifically to human tumor necrosis factor (TNF).
Similarly, by binding specifically to TNF, etanercept binds and blocks its interaction with the cell surface’s TNF receptors. It is produced by recombinant technology in Chinese hamster ovaries and is not an antibody.
Adalimumab, certolizumab, and golimumab are recombinant human monoclonal antibodies that bind to TNF with high affinity. Certolizumab is unique in that it is a pegylated (polyethylene glycolated) Fab fragment derived from a high-affinity humanized anti-TNF monoclonal Ab. The Fab fragments lack the Fc portion of immunoglobulin, so the Fc responses such as complement or Ab-dependent cell-mediated cytotoxicity are not realized, which is distinct from the other anti-TNF Ab, but it still neutralizes membrane anti-TNF.
Patient instructions
Patients should not receive live vaccines during treatment. Therapy should be temporarily discontinued in the event of an acute infection.
Adverse drug events
Therapy has been associated with serious mycobacterial, fungal, and opportunistic infectious complications such as sepsis and tuberculosis, leading to requirement of an FDA black box warning to that effect in 2008. Other adverse reactions include rash, headache, nausea, and cough. Although rare, anti-TNF drugs have been associated with nerve damage that resembles the disease process in multiple sclerosis, congestive heart failure, skin cancers, and lupus-like syndromes. Lymphoma has been reported with TNF antagonists, although risk of solid tumors appears neutral. These drugs should not be used in patients with hepatitis B.
Drug–drug interactions
Live vaccines may interact with these drugs. Biologic drugs should not be used in combination because that increases the risk of infection.
Parameters to monitor
Be clinically alert for tuberculosis, histoplasmosis, and other opportunistic infections.
Other aspects
Patients should be tested for tuberculosis (skin testing, chest radiograph, or both) and hepatitis B (if risk factors are present) before initiating therapy with any biologic agent. Currently, infliximab is approved for therapy only in combination with methotrexate. Patients should receive appropriate vaccinations prior to initiation, such as pneumococcal, influenza, hepatitis B, and herpes zoster.
Dose
The dosage for infliximab is 3 mg/kg intravenous (IV) initially, at weeks 2 and 6, and then every 8 weeks in combination with methotrexate.
The dosage for etanercept is 25 mg subcutaneous twice weekly or 50 mg subcutaneous once weekly.
The dosage for adalimumab is 40 mg subcutaneous every second week.
The dosage for golimumab is 50 mg subcutaneous every 4 weeks.
The dosage for certolizumab is 400 mg subcutaneous initially and at weeks 2 and 4, followed by a dose of 200 mg every other week. Maintenance dosing up to 400 mg every 4 weeks can be considered.
Anakinra
Mechanism of action
Anakinra (Kineret) blocks the biologic activity of interleukin-1 by competitively inhibiting interleukin-1 binding to the interleukin-1 type I receptor.
Patient instructions
Kineret is supplied in a single-use, prefilled syringe that should be stored in the refrigerator. Any syringe left unrefrigerated for more than 24 hours should be discarded.
Adverse drug events
Like the anti-TNF agents, anakinra increases the risk of serious infections. Injection-site reactions are extremely common. Headache, nausea, diarrhea, sinusitis, flu-like symptoms, and abdominal pain have also been reported.
Drug–drug interactions
Live vaccines can interact with anakinra.
Parameters to monitor
Patients should have a CBC checked at baseline, then monthly for 3 months, and then once every 3 months for the first year of therapy.
Dose
The dose is 100 mg subcutaneous daily.
Abatacept
Mechanism of action
Abatacept (Orencia) selectively modulates T-cell activation causing downregulation and an anti-inflammatory effect.
Adverse drug events
Like the other biologic DMARDs, abatacept increases the risk of infections, especially upper respiratory infections. Nausea and headache are also frequently reported. In addition, patients with chronic obstructive pulmonary disease developed adverse effects more frequently than with a placebo. More cases of lung cancer were observed in patients treated with abatacept than with a placebo. The lymphoma rate was higher as well.
Drug–drug interactions
Use of abatacept is contraindicated with other biologic DMARDs because of increased risk of infection. Live vaccines are contraindicated as well.
Other aspects
Abatacept contains maltose and may falsely elevate blood glucose readings. Monitors that do not react to maltose, such as those based on glucose dehydrogenase nicotine adenine dinucleotide, glucose oxidase, or glucose hexokinase test methods, are recommended.
Dose
Dose is based on weight (< 60 kg = 500 mg; 60–100 kg = 750 mg; > 100 kg = 1,000 mg). Infusions are given over 30 minutes. After the initial dose, give at 2 and 4 weeks, followed by every 4 weeks. Alternatively, 125 mg subcutaneous weekly may be prescribed after one weight-based loading dose.
Rituximab
Mechanism of action
Rituximab (Rituxan) causes a transient depletion of B-lymphocytes by binding to the CD20 surface antigens.
Other aspects
Rituximab should be used only in patients with moderate to severe RA who have had an inadequate response or a contraindication to anti-TNF products.
Dose
Give 1,000 mg every 2 weeks for two doses; patients should be premedicated with a glucocorticoid to decrease infusion-related reactions.
Tocilizumab
Mechanism of action
Tocilizumab (Actemra) is an interleukin-6 receptor–inhibiting antibody. The FDA approved tocilizumab in 2010 for moderate to severe RA in adults who have not achieved an adequate response to one or more anti-TNF agents, with or without methotrexate. It can be used with other nonbiologic DMARDs.
Patient instructions
Side effects should be reported to the health care provider immediately.
Adverse drug events
Like the anti-TNF agents, tocilizumab increases the risk of serious infections. It also has been reported to rarely cause generalized peritonitis, diverticulitis, lower GI perforation, fistulae, and intra-abdominal abscesses. Increased transaminases, especially with methotrexate, and decreased white blood cells and platelets have also been reported, as have increased total cholesterol, LDL (low-density lipoprotein), triglycerides, and HDL (high-density lipoprotein) levels. Multiple sclerosis and chronic inflammatory demyelinating polyneuropathy cases may occur, and tocilizumab may carry a malignancy risk of 2.8%.
More common side effects include severe allergic reactions (0.2%), rash (2%), mouth ulcers (2%), abdominal pain (2%), dizziness (3%), hypertension (6%), infusion reactions (7%), headache (7%), upper respiratory infections (5–8%), and serious infections (17.5%).
Drug–drug interactions
In chronic inflammation, the formation of cytochrome P450 (CYP450) enzymes is suppressed by increased levels of cytokines such as interleukin-6. Tocilizumab could normalize the formation of CYP450 enzymes; thus, in hepatically metabolized narrow therapeutic index drugs, an increase in CYP450-mediated metabolism may lower the levels of these drugs (warfarin, theophylline, phenytoin, cyclosporine, carbamazepine, simvastatin, omeprazole, and so on).
Parameters to monitor
■ Patients should have CBC and LFTs checked at baseline, then every 4–8 weeks.
■ Assess lipid parameters at 4–8 weeks following initiation of therapy and every 6 months thereafter. The risk of tuberculosis is not known, but do PPD (purified protein derivative) prior to starting.
Dose
The dose is a 4 mg/kg IV infusion over 1 hour every 4 weeks. The dose may be increased to 8 mg/kg on the basis of clinical response.
Tofacitinib
Mechanism of action
Tofacitinib (Xeljanz) is a janus kinase (JAK) inhibitor. The JAK family (JAK1, JAK2, JAK3, tyrosine kinase 2 [TYK2]) are tyrosine kinase proteins that signal in pairs and facilitate the phosphorylation process of many proteins intracellularly. One such group of proteins is the signal transducers and activators of transcription (STATs). These proteins regulate the transcription of genes that control inflammatory responses. Tofacitinib affects the signaling pathway at the point of the JAK family by preventing phosphorylation and activation of STATs. Tofacitinib is approved for moderate to severe RA patients who have failed or cannot take methotrexate as monotherapy or take tofacitinib in combination with methotrexate or another nonbiologic DMARD. It cannot be combined with another biologic or immunosuppressing drug such as azathioprine or cyclosporine.
Patient instructions
Patients should not receive live vaccines during treatment. Discontinue tofacitinib during infections.
Adverse drug events
Serious infections, increased risk of malignancies, increased lipids, neutropenia, transaminases elevations, and drops in hemoglobin have been reported. One intestinal perforation was reported in clinical trials. A REMS [Risk Evaluation and Mitigation Strategy] Medication Safety Guide is available for patients.
Drug–drug interactions
Tofacitinib is metabolized by CYP3A4; therefore, drugs that inhibit or induce CYP3A4 may affect its pharmacokinetics. Drugs that inhibit CYP2C19 alone or P-glycoprotein are unlikely to affect tofacitinib.
Parameters to monitor
Patients should have baseline CBC, and then hemoglobin again at 4–8 weeks and every 3 months thereafter. Lymphocyte count should be done at baseline and every month. Lipids should be done at 4–8 weeks, and liver tests should be checked periodically. Patients should be vigilant to report any signs or symptoms of infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


