KEY CONCEPTS
![]() Rheumatoid arthritis (RA) is a systemic disease characterized by symmetrical inflammation of joints, yet may involve other organ systems.
Rheumatoid arthritis (RA) is a systemic disease characterized by symmetrical inflammation of joints, yet may involve other organ systems.
![]() Control of inflammation is the key to slowing or preventing disease progression as well as managing symptoms.
Control of inflammation is the key to slowing or preventing disease progression as well as managing symptoms.
![]() Drug therapy should be only part of a comprehensive program for patient management, which would also include physical therapy, exercise, and rest. Assistive devices and orthopedic surgery may be necessary in some patients.
Drug therapy should be only part of a comprehensive program for patient management, which would also include physical therapy, exercise, and rest. Assistive devices and orthopedic surgery may be necessary in some patients.
![]() Disease-modifying antirheumatic drugs (DMARDs) or biologic agents should be started early in the course of the disease and shortly after diagnosis of RA.
Disease-modifying antirheumatic drugs (DMARDs) or biologic agents should be started early in the course of the disease and shortly after diagnosis of RA.
![]() Nonsteroidal antiinflammatory drugs and/or corticosteroids should be considered adjunctive therapy early in the course of treatment and as needed if symptoms are not adequately controlled with DMARDs.
Nonsteroidal antiinflammatory drugs and/or corticosteroids should be considered adjunctive therapy early in the course of treatment and as needed if symptoms are not adequately controlled with DMARDs.
![]() When DMARDs used singly are ineffective or not adequately effective, combination therapy with two or more DMARDs or a DMARD plus biologic agent may be used to induce a response.
When DMARDs used singly are ineffective or not adequately effective, combination therapy with two or more DMARDs or a DMARD plus biologic agent may be used to induce a response.
![]() Patients require careful monitoring for toxicity and therapeutic benefit for the duration of treatment.
Patients require careful monitoring for toxicity and therapeutic benefit for the duration of treatment.
Rheumatoid arthritis (RA) is the most common systemic inflammatory disease characterized by symmetrical joint involvement. Extraarticular involvement, including rheumatoid nodules, vasculitis, eye inflammation, neurologic dysfunction, cardiopulmonary disease, lymphadenopathy, and splenomegaly, can be manifestations of the disease. Although the usual disease course is chronic, some patients will enter a remission spontaneously.
EPIDEMIOLOGY
RA is estimated to have a prevalence of 1% and does not have any racial predilections. It can occur at any age, with increasing prevalence up to the seventh decade of life. The disease is three times more common in women. In people ages 15 to 45 years, women predominate by a ratio of 6:1; the sex ratio is approximately equal among patients in the first decade of life and in those older than age 60 years.
Epidemiologic data suggest that a genetic predisposition and exposure to unknown environmental factors may be necessary for expression of the disease. The major histocompatibility complex molecules, located on T lymphocytes, appear to have an important role in most patients with RA. These molecules can be characterized using human lymphocyte antigen (HLA) typing. A majority of patients with RA have HLA-DR4, HLA-DR1, or both antigens in the major histocompatibility complex region. Patients with HLA-DR4 antigen are 3.5 times more likely to develop RA than those patients who have other HLA-DR antigens.1 Although the major histocompatibility complex region is important, it is not the sole determinant as patients can have the disease without these HLA types. RA is six times more common among dizygotic twins and nontwin children of parents with rheumatoid factor-positive, erosive RA when compared with children whose parents do not have the disease. If one of a pair of monozygotic twins is affected, the other twin has a 30 times greater risk of developing the disease.2,3
PATHOPHYSIOLOGY
![]() Chronic inflammation of the synovial tissue lining the joint capsule results in the proliferation of this tissue. The inflamed, proliferating synovium characteristic of RA is called pannus. This pannus invades the cartilage and eventually the bone surface, producing erosions of bone and cartilage and leading to destruction of the joint. The factors that initiate the inflammatory process are unknown.
Chronic inflammation of the synovial tissue lining the joint capsule results in the proliferation of this tissue. The inflamed, proliferating synovium characteristic of RA is called pannus. This pannus invades the cartilage and eventually the bone surface, producing erosions of bone and cartilage and leading to destruction of the joint. The factors that initiate the inflammatory process are unknown.
The immune system is a complex network of checks and balances designed to discriminate self from nonself (foreign) tissues. It helps rid the body of infectious agents, tumor cells, and products associated with the breakdown of cells. In RA, this system is no longer able to differentiate self from nonself tissues and attacks the synovial and other connective tissues.
In addition to the genetic factors mentioned above, environmental factors play a role. It is known that smoking and pulmonary disease may increase risk. Infectious agents (e.g., Epstein-Barr virus, Escherichia coli) and periodontal disease (Porphyromonas gingivalis) have been associated with RA.
The immune system has both humoral and cell-mediated functions (Fig. 72-1). The humoral component is necessary for the formation of antibodies. These antibodies are produced by plasma cells, which are derived from B lymphocytes. Most patients with RA form antibodies called rheumatoid factors. Rheumatoid factors have not been identified as pathogenic, nor does the quantity of these circulating antibodies always correlate with disease activity. Seropositive patients tend to have a more aggressive course of their illness than do seronegative patients. Anticitrullinated protein antibody (ACPA) is another antibody identified, which is produced in most patients with RA and has become an important diagnostic tool. Patients may develop ACPA long before they develop symptoms of RA, and those with positive antibodies have a poorer prognosis than those without.
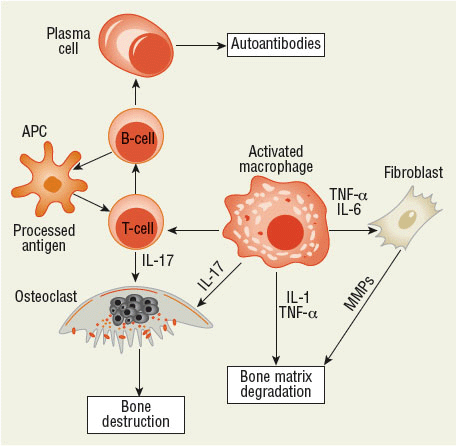
FIGURE 72-1 Pathogenesis of the inflammatory response. Antigen-presenting cells process and present antigens to T cells, which may stimulate B cells to produce antibodies and osteoclasts to destroy and remove bone. Macrophages stimulated by the immune response can stimulate T cells and osteoclasts to promote inflammation. They also can stimulate fibroblasts, which produce matrix metalloproteinases to degrade the bone matrix and produce proinflammatory cytokines. Activated T cells and macrophages release factors that promote tissue destruction, increase blood flow, and result in cellular invasion of synovial tissue and joint fluid. (APC, antigen-presenting cell; IL, interleukin; MMP, matrix metalloproteinase; TNF-α, tumor necrosis factor α.)
The invasion of the synovium and joint by leukocytes results in synovitis. These leukocytes migrate to the region directed by chemokines and adhesion molecules. Early in the inflammatory process, increased vascularity aids in cell trafficking. The synovium proliferates and fibroblasts are activated, and this promotes bone and connective tissue destruction.
Immunoglobulins can activate the complement system. The complement system amplifies the immune response by encouraging chemotaxis, phagocytosis, and the release of lymphokines by mononuclear cells, which are then presented to T lymphocytes. The processed antigen is recognized by major histocompatibility complex proteins on the lymphocyte, which activates it to stimulate the production of T and B cells. The proinflammatory cytokines tumor necrosis factor (TNF), interleukin (IL)-1 and IL-6 are key substances in the initiation and continuance of rheumatoid inflammation. IL-17 can induce proinflammatory cytokines in fibroblasts and synoviocytes and stimulate the release of matrix metalloproteinases and other cytotoxic substances, which leads to cartilage destruction. Activated T cells produce cytotoxins, which are directly toxic to tissues, and cytokines, which stimulate further activation of inflammatory processes and attract cells to areas of inflammation. Macrophages are stimulated to release prostaglandins and cytotoxins.4–6 T-cell activation requires both stimulation by proinflammatory cytokines as well as interaction between cell surface receptors, called costimulation. One of these costimulation interactions is between CD28 and CD80/86. The binding of the CD80/86 receptor by the drug abatacept has proved to be an effective treatment for RA by preventing costimulation interactions between T cells.7
CLINICAL PRESENTATION Rheumatoid Arthritis
Although it has been suggested that T cells play a key role in the pathogenesis of RA, B cells clearly have an equally important role. Evidence for this importance may be found in the effectiveness of B-cell depletion using the drug rituximab in controlling rheumatoid inflammation. Activated B cells produce plasma cells, which form antibodies. These antibodies in combination with the complement system result in the accumulation of polymorphonuclear leukocytes, which release cytotoxins, oxygen-free radicals, and hydroxyl radicals that promote cellular damage to synovium and bone. The benefits of B-cell depletion occur even though antibody formation is not suppressed with rituximab therapy; this suggests that other mechanisms play a role in reducing RA activity. B cells produce cytokines that may alter the function of other immune cells, and they also have the ability to process antigens and act as antigen-presenting cells, which interact with T cells to activate the immune process.8–11
In the synovial membrane, CD4+ T cells are abundant and communicate with macrophages, osteoclasts, fibroblasts, and chondrocytes either through direct cell–cell interactions using cell surface receptors or through proinflammatory cytokines such as TNF-α, IL-1, and IL-6. These cells produce metalloproteinases and other cytotoxic substances, which lead to the erosion of bone and cartilage. They also release substances promoting growth of blood vessels and adhesion molecules, which assists in proinflammatory cell trafficking and attachment of fibroblasts to cartilage and eventual synovial invasion and destruction.12–15 TNF inhibitors are widely used to treat RA. Although anakinra inhibits IL-1 by attaching to receptors on cell surface, the benefits of this approach have not been as great as expected. Tocilizumab has proven effective as an inhibitor of IL-6 activity.
There are also a number of signaling molecules that are important for activating and maintaining inflammation. One of these is Janus kinase (JAK), which is a tyrosine kinase responsible for regulating leukocyte maturation and activation. JAK also has effects on the production of cytokines and immunoglobulins. Tofacitinib, an oral JAK inhibiting drug, has proven to be very effective in RA and appears to inhibit IL-6 activity as the major mechanism of action.
Vasoactive substances also play a role in the inflammatory process. Histamine, kinins, and prostaglandins are released at the site of inflammation. These substances increase both blood flow to the site of inflammation and the permeability of blood vessels. These substances cause the edema, warmth, erythema, and pain associated with joint inflammation and make it easier for granulocytes to pass from blood vessels to the site of inflammation.
The end results of the chronic inflammatory changes are variable. Loss of cartilage may result in a loss of the joint space. The formation of chronic granulation or scar tissue can lead to loss of joint motion or bony fusion (called ankylosis). Laxity of tendon structures can result in a loss of support to the affected joint, leading to instability or subluxation. Tendon contractures also may occur, leading to chronic deformity.12,16
The symptoms of RA usually develop insidiously over the course of several weeks to months. Prodromal symptoms include fatigue, weakness, low-grade fever, loss of appetite, and joint pain. Stiffness and muscle aches (myalgias) may precede the development of joint swelling (synovitis). Fatigue may be more of a problem in the afternoon. During disease flares, the onset of fatigue begins earlier in the day and subsides as disease activity lessens. Most commonly, joint involvement tends to be symmetrical; however, early in the disease some patients present with an asymmetrical pattern involving one or a few joints that eventually develops into the more classic presentation. Approximately 20% of patients develop an abrupt onset of their illness with fevers, polyarthritis, and constitutional symptoms (e.g., depression, anxiety, fatigue, anorexia, and weight loss).2,3
No single test or physical finding can be used to make the diagnosis of RA. In early disease, the diagnosis can be particularly challenging given that radiographic findings are usually absent and rheumatoid factor test can be undetectable. Duration of joint pain and swelling, morning stiffness lasting more than 1 hour, and involvement of three or more joints are important early predictors of the development of persistent erosive RA.17
JOINT INVOLVEMENT
The joints affected most frequently by RA are the small joints of the hands, wrists, and feet (Fig. 72-2). In addition, elbows, shoulders, hips, knees, and ankles may be involved. Patients usually experience joint stiffness that typically is worse in the morning. The duration of stiffness tends to be correlated directly with disease activity, usually exceeds 30 minutes, and may persist all day. Chronic inflammation with lack of an adequate exercise program results in loss of range of motion, atrophy of muscles, weakness, and deformity (Figs. 72-3 and 72-4).
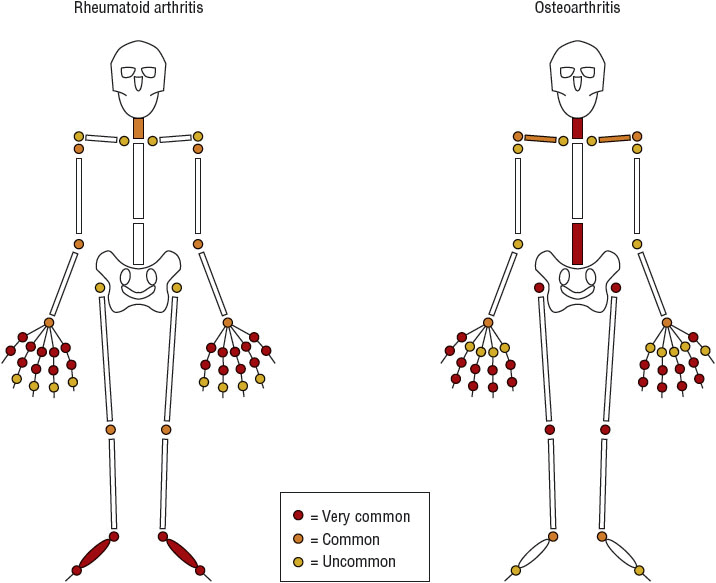
FIGURE 72-2 Patterns of joint involvement in rheumatoid arthritis and osteoarthritis.

FIGURE 72-3 Deformities of rheumatoid arthritis, with marked ulnar deviation, swan-neck deformity, active synovitis, and nodules. (Reproduced with permission from Brunicardi FC, Anderson DK, Billiar TR, et al. Schwartz’s Principles of Surgery, 8th ed. New York: McGraw-Hill, 2005.)
FIGURE 72-4 A. Preoperative view of metacarpophalangeal joints in rheumatoid arthritis. B. Following resection arthroplasty. (Reproduced with permission from Skinner H., ed. Current Diagnosis & Treatment in Orthopedics, 4th ed. New York: McGraw-Hill, 2006:592.)
On examination, the swelling of the joints may be visible or may be apparent only by palpation. The swelling feels soft and spongy because it is caused by proliferation of soft tissues or fluid accumulation within the joint capsule. The swollen joint may appear erythematous and feel warmer than nearby skin surfaces, especially early in the course of the disease. In contrast, the swelling associated with osteoarthritis usually is bony (caused by osteophytes) and infrequently is associated with signs of inflammation.
Involvement of the hands and wrists is common in RA. Hand involvement is manifested by pain, swelling, tenderness, and grip weakness during the acute phase and by subluxation, instability, deformity, and muscle atrophy in the chronic phase of the disease. Functional difficulties with clasp, grasp, and pinch alter both strength and fine motor movement.
Deformity of the hand may be seen with chronic inflammation. These changes may alter the mechanics of hand function, reducing grip strength and making it difficult to perform usual daily activity.
Pain in the elbow and shoulder may be the result of true joint inflammation or inflammation of soft-tissue structures such as tendons (tendonitis) or the bursa (bursitis). The knee also can be involved, with loss of cartilage, instability, and joint pain. Synovitis of the knee may cause the formation of a cyst behind the knee called a popliteal or Baker’s cyst. These cysts may become painful as they get tense, or they may rupture, producing a clinical picture similar to thrombophlebitis secondary to the release of inflammatory components into the area of the calf muscle (pseudothrombophlebitis syndrome). Chronic joint pain leads to muscle atrophy, which can result in a laxity of the ligamentous structures that support the knee, causing instability. Maintenance of an adequate range of motion of the knee is essential to normal gait.
Foot and ankle involvement in RA is common. The metatarsophalangeal joints are involved frequently in RA, making walking difficult. Subluxation of the metatarsal heads leads to “cock-up” or hammer toe deformities. Subluxation also may cause a flexion deformity at the proximal interphalangeal joint of the toe, leading to pressure necrosis of the skin over the joint secondary to irritation caused by shoes. Hallux valgus (lateral deviation of the digit) and bunion or callus formation may occur at the great toe. A widening of the foot occurs commonly with long-standing disease.
Involvement of the spine usually occurs in the cervical vertebrae; lumbar vertebral involvement is rare. Involvement of the first and second cervical vertebrae (C1 to C2) can lead to instability of this joint. Patients with this problem are at a greater risk for spinal cord compression, although this complication is rare.
The temporomandibular joint (jaw) can be affected, resulting in malocclusion and difficulty in chewing food. Inflammation of cartilage in the chest can lead to chest wall pain. Hip pain may occur as a result of destructive changes in the hip joint, soft-tissue inflammation (e.g., bursitis), or referred pain from nerve entrapment at the lumbar vertebrae.
EXTRAARTICULAR INVOLVEMENT
Although joint involvement in RA is a hallmark finding in RA, it is important to recognize that, as a systemic disease, other organ systems are often involved.
Rheumatoid Nodules
Rheumatoid nodules occur in 20% of patients with RA. These nodules are seen most commonly on the extensor surfaces of the elbows, forearms, and hands but also may be seen on the feet and at other pressure points. They also may develop in the lung or pleural lining of the lung and, rarely, in the meninges. Rheumatoid nodules usually are asymptomatic and do not require any special intervention. Nodules are observed more commonly in patients with erosive disease.18
Vasculitis
Vasculitis usually is seen in patients with long-standing RA. Vasculitis may result in a wide variety of clinical presentations. Invasion of blood vessel walls by inflammatory cells results in an obliteration of the vessel, producing infarction of tissue distal to the area of involvement. Most commonly, small-vessel vasculitis produces infarcts near the ends of the fingers or toes, especially around the nail beds. These infarcts are usually of little consequence.
Vasculitis also may cause the breakdown of skin, especially in the lower extremities, producing ulcers that may be indistinguishable in appearance from stasis ulcers. However, these ulcers do not heal with the usual modes of treatment used for stasis ulcers. Involvement of larger vessels with vasculitis can result in life-threatening complications. Infarction of vessels supplying blood to nerves can cause irreversible motor deficits. Involvement of vessels supplying other organ systems can lead to visceral involvement and a polyarteritis nodosa-like illness. Aggressive treatment of the inflammatory process is necessary in these patients. Fortunately, vasculitis has become much less frequently seen since the advent of methotrexate and biologic therapy.
Pulmonary Complications
RA may involve the pleura of the lung, which is often asymptomatic, although pleural effusions may result. Pulmonary fibrosis also may develop as a result of rheumatoid involvement; smoking appears to increase the risk of this complication. Rheumatoid nodules may develop in lung tissue and appear similar to neoplasms on chest radiographs. Interstitial pneumonitis and arteritis are rare, potentially life-threatening complications of RA.
Ocular Manifestations
Ocular manifestations include keratoconjunctivitis sicca and inflammation of the sclera, episclera, and cornea. Atrophy of the lacrimal duct may result in a decrease in tear formation, causing dry and itchy eyes, termed keratoconjunctivitis sicca. When this is observed in association with RA, it is referred to as Sjögren’s syndrome. Artificial tears may be used to relieve symptoms. The salivary glands may also be involved in Sjögren’s syndrome, resulting in dry mouth (xerostomia). Inflammation of the superficial layers of the sclera (episcleritis) is generally self-limiting. Involvement of deeper tissues (scleritis) usually results in a more serious, painful, and chronic inflammation. Rheumatoid nodules may develop on the sclera.
Cardiac Involvement
The heart is sometimes affected by RA. RA is associated with an increased risk of cardiovascular mortality. This risk appears to be higher in those with more active inflammation and is reduced with treatment, particularly with methotrexate.19,20 Pericarditis may occur, resulting in the accumulation of fluid. Although many patients show evidence of previous pericarditis at autopsy, the development of clinically evident pericarditis with tamponade is a rare complication. Cardiac conduction abnormalities and aortic valve incompetence, caused by aortic root dilation, may occur. Myocarditis is a rare complication of RA.
Felty’s Syndrome
RA in association with splenomegaly and neutropenia is known as Felty’s syndrome. Thrombocytopenia also may be a manifestation of the syndrome. Patients with Felty’s syndrome and severe leukopenia are more susceptible to infection. The decrease in granulocytes appears to be mediated by the immune system because splenectomy does not result in improvement of the patient.18
Other Complications
Lymphadenopathy may occur in patients with RA, particularly in nodes proximal to more actively involved joints. Renal involvement is rare but can be associated with treatment, including nonsteroidal antiinflammatory drugs (NSAIDs), gold salts, and penicillamine. Amyloidosis is a rare complication of longstanding RA. It appears to be more common in Europe than in the United States.
LABORATORY FINDINGS
Hematologic tests often reveal a mild-to-moderate anemia with normocytic, normochromic indices. The hematocrit may fall as low as 30%. The anemia is usually inversely related to inflammatory disease activity and is referred to as an anemia of chronic disease. This type of anemia does not respond to iron therapy and can present a diagnostic dilemma because NSAIDs may induce gastritis and chronic blood loss, leading to iron-deficiency anemia. Laboratory tests useful in differentiating these anemias include stool guaiac (or other stool tests for occult blood), serum iron-to-iron-binding capacity ratio (decreased in iron deficiency), ferritin (decreased in iron deficiency), and mean corpuscular volume (more likely to be decreased in iron deficiency). Other causes of anemia also must be considered in the differential diagnosis (see Chaps. 80 and 82).
Thrombocytosis is another common hematologic finding with active RA. Platelet counts rise and fall in direct correlation with disease activity in many patients. Thrombocytopenia may result from toxicity of immunosuppressive therapy. Thrombocytopenia also may be observed in Felty’s syndrome or vasculitis.
Although leukopenia is associated with Felty’s syndrome, it also may result from toxicity of methotrexate, gold, sulfasalazine, penicillamine, and immunosuppressive drugs. Leukocytosis is seen commonly as a result of corticosteroid treatment.
The erythrocyte sedimentation rate (ESR) is usually elevated in patients with RA and other inflammatory diseases. This test is very nonspecific, and although the ESR usually falls as patients respond to therapy, there is a large variability among patients in response to treatment. C-reactive protein (CRP) is another nonspecific marker for inflammatory arthritis when it is elevated. This protein is produced by the liver in response to certain cytokines.
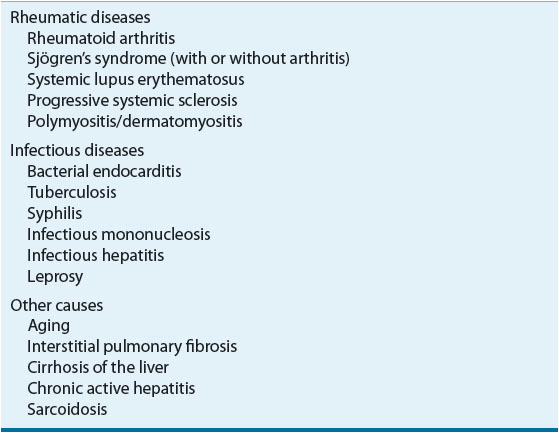
Rheumatoid factor (RF) is present in 60% to 70% of patients with RA. The usual laboratory test for RF is an antibody specific for immunoglobulin (Ig)M RF. Patients with RA and a negative test for RF may have IgG or IgA RFs, but tests for these are not routinely available. RF tests may be reported positive at a specific serum dilution. Serum is diluted to a standard series of dilutions; the greatest dilution that yields a positive test result will be reported (e.g., RF positive at 1:640). Some laboratories quantify RF rather than using titers. Higher dilutional titers or serum concentrations of RFs usually indicate a more severe disease, but like the ESR, the large interpatient variability makes this test unreliable as a means of assessing patient progress. RF may be positive in patients without RA (Table 72-1).
TABLE 72-1 Diseases Associated with a Positive Rheumatoid Factor
ACPA has similar sensitivity for RA, being found in 50% to 85% of patients with the disease, but is more specific (90% to 95%) and is detectable very early in the disease. Many rheumatologists will do both tests in evaluating new patients.
Antinuclear antibodies (ANAs) are detected in 25% of patients with RA. These antibodies usually have a diffuse pattern of immunofluorescence. Tests for antibodies to double-stranded DNA (usually positive in systemic lupus erythematosus) are negative. Serum complement is usually normal, although complement concentrations of joint fluid often are depressed from consumption secondary to the inflammatory process. In patients with vasculitis, serum complement concentrations may be low.21,22
Synovial fluid usually is turbid because of the large number of leukocytes in inflammatory fluid. White cell counts of 5,000 to 50,000/mm3 (5 × 109 to 50 × 109/L) are not uncommon in inflamed joints. The fluid is usually less viscous than that in normal joints or fluid associated with osteoarthritis. Glucose concentrations of joint fluid are normal or low compared with those in serum drawn at the same time as synovial aspirates. The decrease is not as profound as the decrease associated with joint infection or systemic lupus erythematosus.
Early radiographic manifestations of RA include soft-tissue swelling and osteoporosis near the joint (periarticular osteoporosis). Joint space narrowing occurs as a result of cartilage degradation. Erosions tend to occur later in the course of the disease and usually are seen first in the metacarpophalangeal and proximal interphalangeal joints of the hands and the metatarsophalangeal joints of the feet. Periodic joint radiographs are a useful way of evaluating disease progression.
Diagnostic Criteria
The American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) revised criteria for the diagnosis of RA.23 These criteria were developed to be used for patients early in their disease; they, therefore, emphasize early manifestations of the disease. Late manifestations of RA such as erosive disease or nodules are no longer in the diagnostic criteria, but these patients would have previously met these criteria based on retrospective data.
Patients with synovitis of at least one joint and no other explanation for the finding are candidates for these criteria. The criteria use a scoring system with a score of >6 out of a possible total score of 10 as being diagnostic for RA. More points are given for patients presenting with more actively involved joints. Positive laboratory tests including RF, ACPA, CRP, and ESR result in additional points.
Duration of symptoms ≥6 weeks results in an additional point. It is important to note that not all patients with RA may have a score >6 initially, particularly if seen very early in their disease but may evolve to higher scores over time. Reassessment should be considered for those with ongoing symptoms.
Seronegative Inflammatory Arthritis
Although RA may have a negative RF titer, a number of other systemic inflammatory arthritic conditions exist including psoriatic arthritis, reactive arthritis, ankylosing spondylitis, and arthritis associated with inflammatory bowel disease. These conditions often tend to be less aggressive than what is typically seen with RA. Detailed discussion about these conditions is beyond the scope of this chapter, but further information may be found elsewhere.2 Management principles are similar to those for RA.
TREATMENT
Rheumatoid Arthritis
Desired Outcome
![]() The primary objective in the treatment of RA is to improve or maintain functional status, thereby improving quality of life. The ultimate goal is to achieve complete disease remission or low disease activity, although this goal may not be possible to achieve in some patients. Additional goals of treatment include controlling disease activity and joint pain, maintaining the ability to function in daily activities or work, slowing destructive joint changes, and delaying disability.
The primary objective in the treatment of RA is to improve or maintain functional status, thereby improving quality of life. The ultimate goal is to achieve complete disease remission or low disease activity, although this goal may not be possible to achieve in some patients. Additional goals of treatment include controlling disease activity and joint pain, maintaining the ability to function in daily activities or work, slowing destructive joint changes, and delaying disability.
General Approach to Treatment
The multifaceted treatment approach includes pharmacologic and nonpharmacologic therapies with recent emphasis being placed on aggressive treatment early in the disease course. Early aggressive treatment may prevent irreversible joint damage and disability. As many pharmacologic agents are available for the treatment of RA, the recommended drug therapy is based on disease duration, activity, and prognosis.24,25 In general, patients with less active disease and good prognostic indicators may be treated with oral agents as monotherapy. Those with high disease activity and/or poor prognostic features are candidates for combination therapy and biologics to suppress inflammation. Controlling inflammation with therapeutic interventions improves symptoms, slows the disease course, and prevents disease progression.
Nonpharmacologic Therapy
![]() Rest, occupational therapy, physical therapy, use of assistive devices, weight reduction, and surgery are the most useful types of nonpharmacologic therapy used in patients with RA. Rest is an essential component of a nonpharmacologic treatment plan. It relieves stress on inflamed joints and prevents further joint destruction. Rest also aids in alleviation of pain. Too much rest and immobility, however, may lead to decreased range of motion and, ultimately, muscle atrophy, and contractures.
Rest, occupational therapy, physical therapy, use of assistive devices, weight reduction, and surgery are the most useful types of nonpharmacologic therapy used in patients with RA. Rest is an essential component of a nonpharmacologic treatment plan. It relieves stress on inflamed joints and prevents further joint destruction. Rest also aids in alleviation of pain. Too much rest and immobility, however, may lead to decreased range of motion and, ultimately, muscle atrophy, and contractures.
Occupational and physical therapy can provide the patient with skills and exercises necessary to increase or maintain mobility. These disciplines may also supply patients with supportive and adaptive devices such as canes, walkers, and splints.
Other nonpharmacologic therapeutic options include weight loss and surgery. Weight reduction helps to alleviate stress on inflamed joints. This should be instituted and monitored with close supervision of a healthcare professional. Tenosynovectomy, tendon repair, and joint replacements are surgical options for patients with RA. Such management is reserved for patients with severe disease.26–27
Pharmacologic Therapy
![]()
![]() Pharmacologic agents that reduce RA symptoms and impede radiographic joint damage can be categorized as either nonbiologic disease-modifying antirheumatic drugs (DMARDs) or biologic DMARDs, which include TNF-α inhibitor biologics or non-TNF biologics. DMARDs are a treatment cornerstone and should be started as soon as possible after disease onset as early introduction results in more favorable outcomes28–30 and reduces mortality rates comparable to patients without the disease.19,31 NSAIDs and/or corticosteroids may be used for symptomatic relief if needed. They provide relatively rapid improvement in symptoms compared with DMARDs, which may take weeks to months before benefit is seen; however, NSAIDs have no impact on disease progression and the long-term complication risks of corticosteroids make them less desirable.28
Pharmacologic agents that reduce RA symptoms and impede radiographic joint damage can be categorized as either nonbiologic disease-modifying antirheumatic drugs (DMARDs) or biologic DMARDs, which include TNF-α inhibitor biologics or non-TNF biologics. DMARDs are a treatment cornerstone and should be started as soon as possible after disease onset as early introduction results in more favorable outcomes28–30 and reduces mortality rates comparable to patients without the disease.19,31 NSAIDs and/or corticosteroids may be used for symptomatic relief if needed. They provide relatively rapid improvement in symptoms compared with DMARDs, which may take weeks to months before benefit is seen; however, NSAIDs have no impact on disease progression and the long-term complication risks of corticosteroids make them less desirable.28
DMARDs and biologic agents slow RA disease progression. DMARDs commonly used include methotrexate, hydroxychloroquine, sulfasalazine, and leflunomide. The biologic agents with disease-modifying activity include the anti-TNF drugs (etanercept, infliximab, adalimumab, certolizumab, golimumab, tofacitinib), the costimulation modulator abatacept, the IL-6 receptor antagonist tocilizumab, and rituximab, which depletes peripheral B cells. Agents less frequently used because of reduced efficacy, greater toxicity, or both include the IL-1 receptor antagonist anakinra, azathioprine, D-penicillamine, gold (including auranofin), minocycline, cyclosporine, and cyclophosphamide.
DMARDs, either as monotherapy or in combination, are first-line agents for most patients with RA. The order in which these agents are used is not clearly defined, although methotrexate is often chosen because long-term data suggest superior outcomes with methotrexate than with other DMARDs. There is also good documentation for better outcomes with methotrexate in combination therapy if methotrexate monotherapy does not achieve an adequate response. Leflunomide appears to have similar long-term efficacy as that of methotrexate.32
![]() Combination therapy with two or more DMARDs may be effective when single-DMARD treatment is unsuccessful.29,33–35 One study suggests that initial combination therapy with either methotrexate, sulfasalazine plus prednisone, or infliximab plus methotrexate was superior to more conventional sequential monotherapy or step-up combinations of DMARDs in early RA.33 For patients with moderate-to-high disease activity, ACR recommends dual DMARD combinations of methotrexate plus hydroxychloroquine, methotrexate plus leflunomide, or methotrexate plus sulfasalazine. They also recommend the triple combination of sulfasalazine, hydroxychloroquine, and methotrexate.24
Combination therapy with two or more DMARDs may be effective when single-DMARD treatment is unsuccessful.29,33–35 One study suggests that initial combination therapy with either methotrexate, sulfasalazine plus prednisone, or infliximab plus methotrexate was superior to more conventional sequential monotherapy or step-up combinations of DMARDs in early RA.33 For patients with moderate-to-high disease activity, ACR recommends dual DMARD combinations of methotrexate plus hydroxychloroquine, methotrexate plus leflunomide, or methotrexate plus sulfasalazine. They also recommend the triple combination of sulfasalazine, hydroxychloroquine, and methotrexate.24
The anti-TNF and non-TNF biologic agents have proven effective for patients who fail treatment with other DMARDs and were previously reserved for this population subset, partly due to cost. However, ACR now endorses the use of anti-TNF biologics in patients with early disease of high activity and presence of poor prognostic factors, regardless of previous DMARD use.24 Features of poor prognosis include functional limitation, extraarticular disease (e.g., rheumatoid nodules, vasculitis), positive RF or ACPA, or bone erosions. Anti-TNF biologics can be used as either monotherapy or in combination with other DMARDs.25 Use of biologics in combination with methotrexate is more effective than biologic monotherapy and the combination is frequently used. Infliximab, specifically, should be given in combination with methotrexate to prevent development of antibodies that may reduce drug efficacy or induce allergic reactions.24
ACR published recommendations for use of nonbiologic and biologic DMARDs in 2008 and updated them in 2012. These recommendations are not intended to be prescriptive but provide guidance for treatment choice. Recommendations are given based on disease duration, degree of disease activity, and likely prognosis. The recommendations take into account barriers to treatment, including cost and insurance restrictions, by suggesting treatment options with and without expensive biologic agents. Simplified algorithms summarizing these treatment recommendations are provided in Figures 72-5 and 72-6. For more details, see the published recommendations.24,25
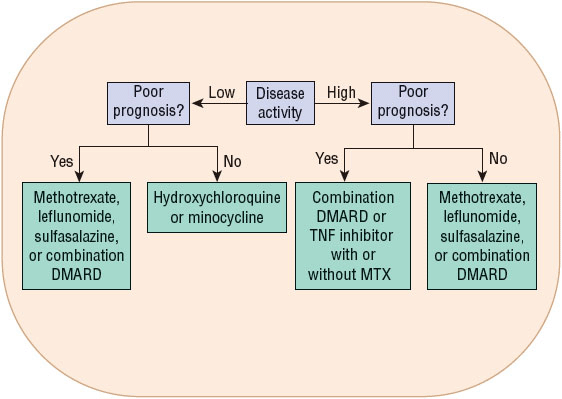
FIGURE 72-5 Algorithm for treatment of rheumatoid arthritis (RA) in early RA (<6 months). Poor prognosis is defined as limitation in function, extraarticular findings (rheumatoid nodules, vasculitis, Felty’s syndrome, Sjögren’s syndrome, rheumatoid lung findings, erosions on radiograph), bone erosions, and positive rheumatoid factor or anticitrullinated protein antibody. (DMARD, disease-modifying antirheumatic drug; MTX, methotrexate; NSAID, nonsteroidal antiinflammatory drug.)



