FIGURE 18-1. Schematic of the antibody molecule. (Reproduced, with permission, from Diamond B, Grimaldi C. B cells. In: Firestein GS, Budd RC, Harris ED Jr, et al., eds. Kelley’s Textbook of Rheumatology. 8th ed. Philadelphia, PA: Saunders Elsevier; 2009:178.)
Both types of chains have a variable region (VL and VH) and a constant region (CL and CH). The variable regions contain the antigen-binding sites and vary in amino acid sequence. The sequences differ to allow immunoglobulins to recognize and bind specifically to thousands of different antigens. Within the variable regions, there are four framework regions (FWR) and three complementarity-determining regions (CDR); together these make up the antigen-binding pocket. The constant region of the light chain (CL) is a single section. Immunoglobulins that have identical constant regions in their heavy chains (e.g., CH1, CH2, and CH3) are of the same class.
The five classes of immunoglobulins are IgA, IgD, IgE, IgG, and IgM. Depending on the immunoglobulin, the constant region of the heavy chain has either three domains and a hinge region (IgA, IgD, and IgG) that promotes flexibility, or four domains without a hinge region (IgE and IgM). Thus, the immunoglobulin’s heavy chain determines its class (alpha heavy chains, IgA; delta heavy chains, IgD; epsilon heavy chains, IgE; gamma heavy chains, IgG; and mu heavy chains, IgM). Tests are available to measure the serum concentrations of the general types of immunoglobulins as well as immunoglobulins directed against specific antigens (viruses, other infectious agents, other allergens).
In Figure 18-1, the second and third domains (CH2 and CH3) of the heavy chain are part of the Fc (fraction crystallizable) portion of the immunoglobulin. This portion has two important functions: (1) activation of the complement cascade (discussed later); and (2) binding of immunoglobulins (which react with and bind antigen) to cell surface receptors of effector cells such as monocytes, macrophages, neutrophils, and natural killer (NK) cells.1
TESTS TO DIAGNOSE AND ASSESS RHEUMATIC DISEASES
Blood tests that are relatively specific for certain rheumatic diseases include rheumatoid factors (RFs), anticyclic citrullinated peptide (anti-CCP) antibodies, antinuclear antibodies (ANAs), antineutrophil cytoplasmic antibodies (ANCAs), and complement. Nonspecific blood and other types of tests include erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), analysis of synovial fluid, and others.
Where applicable, the sections that follow discuss quantitative assay results (where normal values are reported as a range of concentrations), qualitative assay results (where assay results are reported as only positive or negative), and their use in common rheumatic and nonrheumatic diseases.
Rheumatoid Factor
Rheumatoid factors (RFs) are immunoglobulins that are abnormally directed against the Fc portion of IgG. These immunoglobulins do not recognize the IgG as being “self.” Therefore, the presence of RFs in the blood indicates an autoimmune process. The RF measured in most laboratories is IgM-anti-IgG (an IgM antibody that specifically binds IgG). Like all IgM antibodies, IgM RF is composed of five subunits whose Fc portions are attached to the same base. The variable regions of each IgM antibody can bind up to five IgG molecules at its multiple binding sites, making IgM RF the most stable and easiest to quantify.
Rheumatoid factors are most commonly associated with rheumatoid arthritis (RA) but are not specific for that disease. Other rheumatic diseases in which circulating RFs have been identified include systemic lupus erythematosus (SLE), systemic sclerosis (scleroderma), mixed connective tissue disease (MCTD), and Sjögren syndrome.2 The significance of RFs in these diseases is unknown.
The presence of RF is not conclusive evidence that a rheumatic disease exists. Patients with various acute and chronic inflammatory diseases as well as healthy individuals may be RF positive. Nonrheumatic diseases associated with RFs include mononucleosis, hepatitis, malaria, tuberculosis, syphilis, subacute bacterial endocarditis, cancers after chemotherapy or irradiation, chronic liver disease, hyperglobulinemia, and cryoglobulinemia.
The percentage of individuals with positive RF concentrations and the mean RF concentration of the population increase with advancing age. Although RFs are associated with several rheumatic and many nonrheumatic diseases, the concentrations of RFs in these diseases are lower than those observed in patients with RA.
Quantitative Assay Results
Normal values: <1:80 or less than 40–60 International Units/mL
When a quantitative RF test is performed, results are reported as either a dilutional titer or a concentration in International Units per milliliter. Rheumatoid factor titers are reported positive as a specific serum dilution. That is, serum is diluted serially (e.g., 1:20, 1:40, 1:80, 1:160, 1:320); the ability to detect RF is tested at each dilution. The greatest dilution that results in a positive test is reported as the endpoint. A titer of >1:80 or a concentration >40–60 International Units/mL is generally considered to be positive.
Qualitative Assay Results
The dilutional titer chosen to indicate a positive RF excludes 95% of the normal population. Stated another way, at a serum dilution at which 95% of the normal population is RF negative, 70% to 90% of RA patients will have a positive RF test. The remaining RA patients who have RF titers within the normal range may be described as seronegative.
Anticyclic Citrullinated Peptide (anti-CCP) Antibodies
The anti-CCP test is also known as anticitrullinated protein antibody (ACPA) or citrulline antibody. This antibody binds to the nonstandard amino acid citrulline that is formed from removal of amino groups from arginine. Nonstandard amino acids are generally not found in proteins and often occur as intermediates in the metabolic pathways of standard amino acids. In the joints of RA patients, proteins may be transformed to citrulline during the process that leads to joint inflammation. The anti-CCP antibody is present in most patients with RA but is found much less often in patients with other diseases. The test is highly specific for RA; when these antibodies are present, there is a 90% to 95% likelihood that the patient has RA. The combination of both positive RF and positive anti-CCP antibody has 99.5% specificity for RA.
The anti-CCP test is most useful in helping to identify the etiology of inflammatory arthritis in patients with negative RF titers. Anti-CCP antibodies are detected in about 50% to 60% of patients with early RA, usually after 3–6 months of symptoms.3 It has been theorized that citrulline antibodies represent the earlier stages of RA in this situation. The presence of anti-CCP antibodies has also been associated with more erosive forms of RA. Therefore, research is needed to determine whether anti-CCP–positive patients with early stage RA disease benefit from aggressive treatment at an early stage of disease.
Quantitative and Qualitative Assay Results
Normal values: <20 EU/mL (assay dependent)
Quantitative anti-CCP antibodies are tested by enzyme-linked immunosorbent assay (ELISA) and are reported in ELISA units (EU). The relationship between these values and qualitative results are generally reported as (1) <20 EU: negative; (2) 20–39 EU: weakly positive; (3) 40–59 EU: moderately positive; and (4) >60 EU: strongly positive.
Antinuclear Antibodies
Antinuclear antibodies (ANAs) are a heterogeneous group of autoantibodies directed against nucleic acids and nucleoproteins within the nucleus and cytoplasm. Intracellular targets of these autoantibodies include deoxyribonucleic acid (DNA), ribonucleic acid (RNA), individual nuclear histones, acidic nuclear proteins, and complexes of these molecular elements (Table 18-1).4-7
ACA = anticentromere antibody; ANA = antinuclear antibody; CREST = syndrome characterized by calcinosis, Raynaud disease, esophageal motility disorder, sclerodactyly, and telangiectasias; DNA = deoxyribonucleic acid; dsDNA = double-stranded DNA; La/SSB = La/Sjögren syndrome B antibody; MCTD = mixed connective tissue disease; RA = rheumatoid arthritis; RNP = ribonucleoprotein; Ro/SSA = Ro/Sjögren syndrome A antibody; SLE = systemic lupus erythematosus; Scl70 = scleroderma-70 or DNA topoisomerase I antibody; Sm = Smith antibody; ssDNA = single-stranded DNA; U1RNP = uridine-rich ribonuclear protein.
aRepresents the first two letters of the surname of the patient whose serum was used to identify the reaction in agar diffusion.
bSjögren syndrome A and B.
Source: From references 4–7.
The ANA test is included in the diagnostic criteria for idiopathic SLE, drug-induced lupus, and MCTD because of its high rate of positivity in these disorders. However, its low specificity makes it unsuitable for use as a screening test for rheumatic or nonrheumatic diseases in asymptomatic individuals. A positive ANA can also be found in otherwise healthy individuals. Antinuclear antibodies are also associated with various genetic and environmental factors (e.g., intravenous drug abuse), hormonal factors, and increased age. They also are associated with nonrheumatic diseases, both immunologically mediated (e.g., Hashimoto thyroiditis, idiopathic pulmonary fibrosis, primary pulmonary hypertension, idiopathic thrombocytopenic purpura, and hemolytic anemia) and nonimmunologically mediated (e.g., acute or chronic bacterial, viral, or parasitic infections; and neoplasm).
Antibody tests that have clinical utility for diagnosis of SLE, drug-induced lupus, and other diseases include the following:
- Double-stranded DNA (dsDNA) antibodies—These antibodies are relatively specific for SLE, which makes them useful for diagnosis of the disorder. In some patients with SLE, the titers tend to rise with a disease flare and fall (usually into the normal range) when the flare subsides. Thus, dsDNA titers may be helpful in managing disease activity in some SLE patients. The dsDNA antibodies have been found in low titers in many other autoimmune diseases (e.g., RA, Sjögren syndrome, systemic sclerosis, Raynaud disease, MCTD, discoid lupus, juvenile rheumatoid arthritis (JRA), and autoimmune hepatitis).6 Presence of dsDNA antibodies has also been reported in patients receiving some drugs used to treat rheumatic diseases (e.g., minocycline, etanercept, infliximab, and penicillamine).
- Single-stranded DNA (ssDNA) antibodies—These antibodies identify and react primarily with purine and pyrimidine bases within the beta helix of dsDNA. They may also bind with nucleosides and nucleotides. They are much less specific for SLE than dsDNA antibodies. Therefore, ssDNA antibodies are of limited usefulness for diagnosing SLE.6 They also do not correlate well with disease activity and are not helpful for managing ongoing disease.
- Smith (Sm) antibodies—These antibodies bind to a series of nuclear proteins complexed with small nuclear RNAs. These complexes are known as small nuclear ribonucleoprotein particles (snRNPs) and are important in the processing of RNA transcribed from DNA.6 The Sm antibody test has low sensitivity (10% to 50% depending on assay methodology) but high specificity (55% to 100%) for SLE. Titers usually remain positive after disease activity has subsided and titers of anti-DNA antibodies have declined to the normal range. Thus, the Sm antibody titer may be a useful diagnostic tool, especially when anti-DNA antibodies are undetectable. There is currently no evidence that monitoring Sm antibodies is useful for following the disease course or predicting disease activity.6
- Ribonucleoprotein (RNP) or uridine-rich ribonuclear protein (U1RNP) antibodies—This antibody system reacts to antigens that are related to Sm antigens. However, these antibodies bind only to the U1 particle, which is involved in splicing nuclear RNA into messenger RNA. Ribonucleoprotein antibodies are found in many patients with SLE (3% to 69%) and low titers may be detected in other rheumatic diseases (e.g., Raynaud disease, RA, systemic sclerosis).6 Importantly, RNP antibodies are a hallmark feature of MCTD. A positive test in a patient with suspected MCTD increases the probability that this diagnosis is correct, even though the test is nonspecific. On the other hand, a negative anti-RNP in a patient with possible MCTD virtually excludes this diagnosis.
- Histone (nucleosome) antibodies—These antibodies target the protein portions of nucleosomes, which are DNA-protein complexes comprising part of chromatin. These antibodies are present in virtually all cases of drug-induced lupus. In fact, the diagnosis of drug-induced lupus should be questioned in their absence. Most cases of drug-induced lupus are readily diagnosed because a commonly implicated drug (e.g., hydralazine, isoniazid, procainamide) is being taken or a strong temporal relationship exists between drug initiation and the onset of SLE signs and symptoms. However, in some cases of potential drug-induced lupus, histone autoantibody testing can be helpful. Histone antibodies appear less commonly in other diseases, including adult RA, juvenile RA, autoimmune hepatitis, scleroderma, and others. There is some evidence that histone antibodies correlate with disease activity in SLE.
Two closely-related ANA tests are detected frequently in patients with Sjögren syndrome, but they are nonspecific; they may also be helpful for diagnosis of SLE.
- Ro/Sjögren syndrome A (Ro/SSA) antibody
- La/Sjögren syndrome B (La/SSB) antibody
The presence of either antibody in patients with suspected Sjögren syndrome strongly supports the diagnosis. It is unusual to detect the La/SSB antibody in patients with SLE or Sjögren syndrome in the absence of the Ro/SSA antibody. In women of childbearing age who have a known connective-tissue disease (e.g., SLE, MCTD), a positive Ro/SSA antibody is associated with an infrequent but definite risk of bearing a child with neonatal SLE and congenital heart block. Presence of the Ro/SSA antibody also correlates with late-onset SLE and secondary Sjögren syndrome. In patients who are ANA negative but have clinical signs of SLE, a positive Ro/SSA antibody may be useful in establishing a diagnosis of SLE.
It has been recommended that the Ro/SSA antibody test be ordered in the following situations7: (1) pregnant women with SLE; (2) women with a history of giving birth to children with heart block or myocarditis; (3) individuals with a history of unexplained photosensitive skin eruptions; (4) patients suspected of having a systemic connective tissue disease with a negative ANA screening test; (5) patients with xerostomia, keratoconjunctivitis sicca, and/or salivary and lacrimal gland enlargement; and (6) patients with unexplained small vessel vasculitis or atypical multiple sclerosis.
Two ANAs are highly specific for systemic sclerosis (scleroderma), but the tests have low sensitivity:
1. Anticentromere antibody (ACA)
2. DNA topoisomerase I (ScI70) antibody—These two antibodies are highly specific for systemic sclerosis and related diseases such as CREST syndrome (associated with calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasias), Raynaud disease, and occasionally SLE. When systemic sclerosis is suspected on clinical grounds, antibody testing for ACA and ScI70 can be useful in making the diagnosis. However, negative results do not exclude the disease because of low test sensitivity.
The Jo-1 antibody (anti-Jo) is highly specific for idiopathic inflammatory myopathy (IIM) including polymyositis and dermatomyositis, or myositis associated with another rheumatic disease or interstitial lung disease.
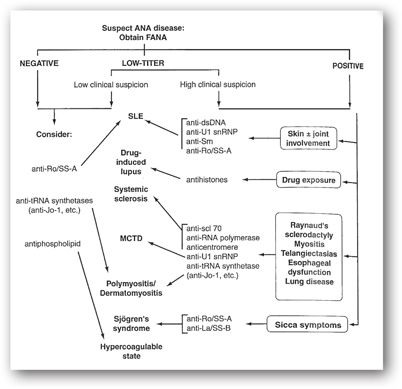
Figure 18-2 provides guidelines for the use of the ANA test in diagnosing rheumatic disorders. The titer or quantitative value should be considered when evaluating the clinical significance of ANA test results.
FIGURE 18-2. Algorithm for the use of ANAs in the diagnosis of connective tissue disorders. (Reproduced, with permission, from Peng ST, Craft JE. Antinuclear antibodies. In: Firestein GS, Budd RC, Harris ED Jr, et al., eds. Kelley’s Textbook of Rheumatology. 8th ed. Philadelphia, PA: Saunders Elsevier; 2009:752.)
Quantitative Antinuclear Antibody Assay Results
Normal: Negative at 1:20 dilution (varies among laboratories)
The indirect immunofluorescence antinuclear antibody test (FANA) is a rapid and highly sensitive method for detecting the presence of ANAs.4 Although the FANA is positive in >95% of patients with SLE, it is also positive in some normal individuals and patients with drug-induced lupus and other autoimmune diseases. An ELISA also provides a rapid and highly sensitive method for detecting the presence of ANA. Many laboratories perform screening ANA tests by the ELISA technique because it can be automated and is less labor intensive; FANA testing is performed only in specimens testing positive by ELISA.5
Laboratories usually report the ANA titer, which is the highest serum dilution that remains positive for ANAs. A very high concentration (titer >1:640) should raise suspicion for an autoimmune disorder but is not in itself diagnostic of any disease. In the absence of clinical findings, these individuals should be monitored closely for the overt development of an autoimmune disorder. On the other hand, a high ANA titer is less useful in a patient who already has definite clinical evidence of a systemic autoimmune disease. The finding of a low antibody titer (<1:80) in the absence of signs or symptoms of disease is not of great concern, and such patients require less frequent followup than those with very high titers. False-positive ANAs are common in the normal population and tend to be associated with low titers (<1:40). The positive antibody titers in healthy persons tend to remain fairly constant over time; this finding can also be seen in patients with known disease.
Qualitative Antinuclear Antibody Assay Results
The pattern of nuclear fluorescence after staining may reflect the presence of antibodies to one or more nuclear antigens. The nuclear staining pattern was used commonly in the past, but pattern type is now recognized to have relatively low sensitivity and specificity for individual autoimmune diseases. For this reason, specific antibody tests have largely replaced use of patterns.5 The common immunofluorescent patterns are as follows:
- Homogeneous—This pattern is seen most frequently in patients with SLE but can also be observed in patients with drug-induced lupus, RA, vasculitis, and polymyositis. This pattern reflects antibodies to the DNA-histone complex.
- Speckled—This pattern is also seen most frequently in SLE but can appear in patients with MCTD, Sjögren syndrome, progressive systemic sclerosis, polymyositis, and RA. This pattern is produced by antibodies to Sm, Ro/SSA, La/SSB, DNA topoisomerase I (ScI70), and other antigens.
- Nucleolar—This pattern is infrequently observed in patients with SLE but is more frequently seen in patients with polymyositis, progressive systemic sclerosis, and vasculitis. It is produced by antibodies to RNA polymerase I and a number of other antigens.
- Peripheral or nuclear rim—This is the only pattern that is highly specific for any rheumatic disease and is observed predominantly (98%) in SLE patients. It is produced by antibodies to DNA (dsDNA, ssDNA) and nuclear envelope antigens (antibodies to components of the nuclear envelope, such as certain glycoproteins).
Table 18-1 summarizes the most frequently identified ANAs, their corresponding targeted cellular material, and disease sensitivities and specificities.4–7
Antineutrophil Cytoplasmic Antibodies
As the name implies, antineutrophil cytoplasmic antibodies (ANCAs) are antibodies directed against neutrophil cytoplasmic antigens. Testing for ANCAs is important for the diagnosis and classification of various forms of vasculitis. In these disorders, the target antigens are proteinase 3 (PR3) and myeloperoxidase (MPO). Both antigens are located in the azurophilic granules of neutrophils and the peroxidase-positive lysosomes of monocytes. Antibodies that target PR3 and MPO are known as PR3-ANCA and MPO-ANCA.8 There is an association between ANCA and several major vasculitic syndromes: Wegener granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome, and certain drug-induced vasculitis syndromes.8
Wegener granulomatosis is a vasculitis of unknown origin that can damage organs by restricting blood flow and destroying normal tissue. Although any organ system may be involved, the disorder primarily affects the respiratory tract (sinuses, nose, trachea, and lungs) and the kidneys. Approximately 90% of patients with active generalized Wegener granulomatosis have ANCAs. In patients with limited disease presentations, up to 40% may be ANCA-negative. Thus, although a positive ANCA test is useful to support a suspected diagnosis, a negative ANCA test does not exclude it. For this reason, the ANCA test is usually not used alone to diagnose this disorder.
In patients with vasculitis, immunofluorescence after ethanol fixation reveals two characteristic patterns: cytoplasmic (cANCA) and perinuclear (pANCA). With cANCA, there is diffuse staining throughout the cytoplasm, which is usually caused by antibodies against PR3. The pANCA pattern is characterized by staining around the nucleus and perinuclear fluorescence. In vasculitis patients, the antibody causing this pattern is generally directed against MPO.
Although detection and identification of ANCAs is most useful in diagnosing various vasculitides, ANCAs have been reported in connective tissue diseases (e.g., RA, SLE, and myositis), chronic infections (e.g., cystic fibrosis, endocarditis, and HIV), and gastrointestinal diseases (e.g., inflammatory bowel disease, sclerosing cholangitis, and autoimmune hepatitis). Some medications may induce vasculitis associated with positive ANCA (usually MPO-ANCA).9 The drugs most strongly associated with ANCA-associated vasculitis are propylthiouracil and methimazole. Hydralazine, minocycline, penicillamine, allopurinol, procainamide, clozapine, phenytoin, rifampin, cefotaxime, isoniazid, and indomethacin are less commonly associated with the disorder.8
When used in the diagnosis of Wegener granulomatosis, the specificity of PR3-ANCA is approximately 90%. The sensitivity of the test is about 90% when the disease is active and 40% when the disease is in remission. Thus, the sensitivity of PR3-ANCA is related to the extent, severity, and activity of the disease at the time of testing.
The utility of obtaining serial PR3-ANCA tests in assessing disease activity is controversial. Some data suggest that a rise in titers predicts clinical exacerbations and justifies increasing immunosuppressive therapy. However, other studies have shown that disease flares cannot be predicted in a timely fashion by elevations in ANCA titers. Further, the immunosuppressive and cytotoxic therapies used are associated with substantial adverse effects. For these reasons, an elevation in ANCA should not be used as the sole justification for initiating immunosuppressive therapy. Rather, patients with rising ANCA titers should be monitored closely with therapy withheld unless there are clear clinical signs of active disease.8
Qualitative Assay Results
The sensitivity and specificity of cANCA, pANCA, and anti-MPO tests for various diseases are listed in Table 18-2. The presence of cANCAs denotes a spectrum of diseases ranging from idiopathic pauci-immune, necrotizing glomerulonephritis to extended Wegener granulomatosis.10 In most cases of vasculitis, renal disorder, and granulomatous disease, patient sera are negative for cANCAs.
cANCA = cytoplasmic antineutrophil cytoplasmic antibody; MPO = myeloperoxidase; pANCA = perinuclear antineutrophil cytoplasmic antibody; RA = rheumatoid arthritis.
The pANCA test has limited diagnostic value. A positive pANCA test should be followed by antigen-specific assays such as anti-MPO. In ulcerative colitis, the specificity of the pANCA test has been reported to be as high as 94%. However, with only moderate sensitivity and inconsistent correlation between titers and disease activity, pANCA screening may be of little value. Although sensitivity can reach 85% in primary sclerosing cholangitis, the pANCA test lacks specificity in the differential diagnosis of autoimmune hepatic diseases. In RA, pANCA may be related to aggressive, erosive disease. The sensitivity of the test increases in RA complicated by vasculitis, but its specificity remains low.
Complement
The complement system consists of at least 17 different plasma proteins that provide a defense mechanism against microbial invaders and serve as an adjunct or “complement” to humoral immunity. The system works by depositing complement components on pathologic targets and by the interaction of plasma proteins in a cascading sequence to mediate inflammatory effects such as opsonization of particles for phagocytosis, leukocyte activation, and assembly of the membrane attack complex (MAC).11 Six plasma control proteins and five integral membrane control proteins regulate this cascade. These proteins circulate normally in a precursor (inactive) form (e.g., C3 and C4). When the initial protein of a given pathway is activated, it activates the next protein (e.g., C3a and C4a) in a cascading fashion similar to that seen with coagulation factors.
Activation of this system can occur through any one of three proteolytic pathways:
- Classical pathway—This pathway is activated when IgM or IgG antibodies bind to antigens such as viruses or bacteria.
- Alternative pathway—This is an evolutionary surveillance system that does not require the presence of specific antibodies.
- Lectin pathway—This pathway is activated similarly to the classical pathway, but instead of antibody binding, mannose-binding protein (MBP) binds to sugar residues on the surface of pathogens.
Activation by any of the three pathways generates enzymes that cleave the third and fifth complement components (C3 and C5). A final common (or terminal) sequence culminates in the assembly of the MAC. Five proteins (C5 through C9) interact to form the MAC, which creates transmembrane channels or pores that displace lipid molecules and other elements, resulting in disruption of cell membranes and cell lysis.
Because the complement system is an important part of immune system regulation, complement deficiency predisposes an individual to infections and autoimmune syndromes. In disorders associated with autoantibodies and the formation of immune complexes, the complement system can contribute to tissue damage.
Serum complement levels reflect a balance between synthesis and catabolism. Hypocomplementemia occurs when the C3 or C4 concentration falls below its reference range. Most cases of hypocomplementemia are associated with hypercatabolism (complement depletion) due to activation of the immune system rather than decreased production of complement components (hyposynthesis). Most diseases associated with the formation of IgG- or IgM-containing circulating immune complexes can cause hypocomplementemia. Rheumatic diseases included in this category are SLE, RA with extra-articular disease, and systemic vasculitis. Nonrheumatic diseases associated with hypocomplementemia include subacute bacterial endocarditis, hepatitis B surface antigenemia, pneumococcal infection, gram-negative sepsis, viral infections (e.g., measles), recurrent parasitic infections (e.g., malaria), and mixed cryoglobulinemia.11
Because errors in interpretation of complement study results can occur, three important aspects should be considered when interpreting these results:
- Reference ranges are relatively wide. Therefore, new test results should be compared with previous test results rather than with a reference range. It is most useful to examine serial test results and correlate changes with a patient’s clinical picture.
- Normal results should be compared with previous results, if available. Inflammatory states may increase the rate of synthesis and elevate serum complement protein levels. For example, some SLE patients have concentrations of specific complement components that are 2–3 times the upper limit of normal (ULN) when their disease is clinically inactive. When the disease activity increases to the point that increased catabolism of complement proteins occurs, levels may then fall into the reference range. It would be a misinterpretation to conclude that these “normal” concentrations represent an inactive complement system. Consequently, serial determinations of complement levels may be more informative than measurements at a single point.
- Complement responses do not correlate consistently with disease activity. In some patients, the increase and decrease of the complement system should not be used to assess disease activity.
Assessment of the complement system should include measurement of the total hemolytic complement activity by the complement hemolytic 50% (CH50) test and determination of the levels of C3 and C4.
Complement Hemolytic 50%
Reference range: 100–250 International Units/mL
The complement hemolytic (CH50) measures the ability of a patient’s serum to lyse 50% of a standard suspension of sheep erythrocytes coated with rabbit antibody. All nine components of the classical pathway are required to produce a normal reaction. The CH50 screening test may be useful when a complement deficiency is suspected or a body fluid other than serum is involved. For patients with SLE and lupus nephritis, serial monitoring of CH50 may be useful for guiding drug therapy.
C3 and C4
Reference ranges: C3, 72–156 mg/dL or 0.72–1.56 g/L; C4, 20–50 mg/dL or 0.2–0.5 g/L
Because C3 is the most abundant complement protein, it was the first to be purified and measured by immunoassay. However, C4 concentrations appear to be more sensitive to smaller changes in complement activation and more specific for identifying complement activation by the classic pathway. Results of C3 and C4 testing are helpful in following patients who initially present with low levels and then undergo treatment, such as those with SLE.
Acute-Phase Reactants
The concentration of a heterogeneous group of plasma proteins, called acute-phase proteins or acute-phase reactants, increases in response to inflammatory stimuli such as tissue injury and infection. Concentrations of CRP, serum amyloid A protein, alpha1-acid glycoprotein, alpha1-antitrypsin, fibrinogen, haptoglobin, prealbumin, ferritin, and complement characteristically increase, whereas serum transferrin, albumin, and transthyretin concentrations decrease. Their collective change is referred to as the acute-phase response.
In general, if the inflammatory stimulus is acute and of short duration, these proteins return to normal within days to weeks. However, if tissue injury or infection is persistent, acute-phase changes may also persist. Additionally, white blood cell (WBC) and platelet counts may be elevated significantly.
Rheumatic diseases are chronic and associated with varying severities of inflammation. The ESR and CRP are two tests that can be helpful in three ways: (1) estimating the extent or severity of inflammation; (2) monitoring disease activity over time; and (3) assessing prognosis.12 Unfortunately, both tests are nonspecific and cannot be used to confirm or exclude any particular diagnosis.
Erythrocyte Sedimentation Rate
Reference range (Westergren method): 0–15 mm/hr for males;
0–20 mm/hr for females
The erythrocyte sedimentation rate (ESR) has been used widely as a reflection of the acute-phase response and inflammation for many years. The test is performed by placing anticoagulated blood in a vertical tube and measuring the rate of fall of erythrocytes in mm/hr. In rheumatic diseases, the ESR is an indirect screen for elevated concentrations of acute-phase plasma proteins, especially fibrinogen.12 An elevated ESR occurs when higher protein concentrations (especially fibrinogen) cause aggregation of erythrocytes, causing them to fall faster.
A number of factors unrelated to inflammation may result in an increased ESR, including obesity, increasing age, and some drugs. The ESR also responds slowly to an inflammatory stimulus. Despite these limitations, the test remains in wide use because it is inexpensive and easy to perform, and a tremendous amount of data is available about its clinical significance in numerous diseases. The Westergren method of performing an ESR test is preferred over the Wintrobe method because of the relative ease of performing the former method in clinical or laboratory settings.
Correlation of serial Westergren ESR results with patient data may influence therapeutic decisions. Two rheumatic diseases, polymyalgia rheumatica and temporal arteritis (giant cell arteritis), are almost always associated with an elevated Westergren ESR. The ESR is usually >60 mm/hr and frequently >100 mm/hr in these disorders. During initial therapy or treatment initiated after a disease flare, a significant decrease or a return to a normal ESR usually indicates that systemic inflammation has decreased substantially. In the absence of clinical symptoms, an increased ESR may indicate that more aggressive therapy is needed. Disease activity can then be monitored by ESR results. Of course, if symptoms are present, they should not be ignored.
C-Reactive Protein
Reference range: 0–0.5 mg/dL or 0–0.005 g/L
C-reactive protein (CRP) is a plasma protein of the acute-phase response. In response to a stimulus such as injury or infection, CRP can increase up to 1000 times its baseline concentration. The precise physiologic function of CRP is unknown, but it is known to participate in activation of the classical complement pathway and interact with cells in the immune system.
Serum CRP levels can be quantitated accurately and inexpensively by immunoassay or laser nephelometry. Most healthy adults have concentrations of <0.3 mg/dL, although concentrations of 1 mg/dL are sometimes seen. Moderate increases range from 1–10 mg/dL, and marked increases are >10 mg/dL.12 Values above 15–20 mg/dL are usually associated with bacterial infections. In general, concentrations >1 mg/dL reflect the presence of a significant inflammatory process. As with the Westergren ESR, serial measurements of CRP are the most valuable, especially in chronic inflammatory diseases.
Currently, the routine use of CRP for the assessment of rheumatic diseases is limited. As with the ESR, CRP concentrations generally increase and decrease with worsening and improving signs and symptoms, respectively. Nevertheless, CRP concentrations are not disease specific, nor are they part of the diagnostic criteria for any rheumatic disease.
Using an assay method called high-sensitivity CRP (hs-CRP), several studies have shown a correlation between elevated levels and cardiovascular events including myocardial infarction. Although controversial, recent research suggests that CRP is simply a marker for atherosclerosis and cardiovascular disease rather than a cause.13 The American Heart Association recommends obtaining hs-CRP levels in patients at intermediate risk of a cardiovascular event (i.e., those whose Framingham multiple risk factor scoring projects a 10-year CHD risk in the range of 10% to 20%).14 In these patients, an elevated CRP (>3 mg/L) is considered to confer high risk. A level of 1–3 mg/L is average risk, and <1 mg/L is low risk. Levels >10 mg/L should be disregarded for coronary risk prediction purposes and the patient should be evaluated for clear sources of systemic inflammation or infection.
It is important to note that the units of measurement for the hs-CRP (mg/L) are different from those of the conventional CRP test (mg/dL). Because CRP levels fluctuate over time, the hs-CRP should be measured twice at least 2 weeks apart and the two values averaged. At the time of this writing, no clinical trials had been conducted to determine whether treating patients on the basis of elevated hs-CRP levels alone is beneficial or cost-effective. Thus, the precise role of hs-CRP testing as a predictor of cardiovascular disease awaits the results of further clinical studies.
Human Leukocyte Antigen B27 (HLA-B27)
Human leukocyte antigen B27 is an antigen on the surface of WBCs encoded by the B locus in the major histocompatibility complex (MHC) on chromosome 6. The HLA-B27 test is qualitative and will be either present or absent. Its presence is associated with autoimmune diseases known as seronegative spondyloarthropathies. An HLA-B27 test may be ordered when a patient has pain and inflammation in the spine, neck, chest, eyes, or joints and an autoimmune disorder associated with the presence of HLA-B27 is the suspected cause. The test may be obtained to confirm a suspected diagnosis of ankylosing spondylitis, Reiter syndrome, or anterior uveitis. However, a positive test cannot distinguish among these diseases and cannot be used to predict progression, severity, prognosis, or the degree of organ involvement. Some patients with these disorders may have a negative HLA-B27 test. Further, the test cannot definitively diagnose or exclude any rheumatologic disease. It is frequently ordered in concert with other rheumatologic tests (e.g., RF, ESR, CRP), based on the clinical presentation.
A positive HLA-B27 in a person without symptoms or a family history of HLA-B27 associated disease is not clinically significant. For example, it does not help predict the likelihood of developing an autoimmune disease. The presence or absence of HLA antigens is genetically determined. If a family member has an HLA-B27 related rheumatologic disease, other family members who share the HLA-B27 antigen have a higher risk of developing a similar disease.
New genetic testing methods permit separation of HLA-B27 into subtypes. Approximately 15 subtypes have been identified; the most common are HLA-B27*05 and HLA-B27*02. The precise clinical significance of individual subtypes is an area of continuing investigation.
Synovial fluid is essentially an ultrafiltrate of plasma to which synovial cells add hyaluronate. This fluid lubricates and nourishes the avascular articular cartilage. Normally, synovial fluid is present in small amounts and is clear and acellular (<200 cells/mm3) with a high viscosity because of the hyaluronic acid concentration. Normal fluid does not clot because fibrinogen and clotting factors do not enter the joint space from the vascular space. Protein concentration is approximately one-third that of plasma, and glucose concentration is similar to that of plasma.
When performing arthrocentesis ( joint aspiration), a needle is introduced into the joint space of a diarthrodial joint. With a syringe, all easily removed synovial fluid is drained from the joint space. Arthrocentesis is indicated as a diagnostic procedure when septic arthritis, hemarthrosis (blood within a joint), or crystal-induced arthritis is suspected. Furthermore, arthrocentesis may be indicated in any clinical situation, rheumatic or nonrheumatic, if the cause of new or increased joint inflammation is unknown. Arthrocentesis is also performed to administer intra-articular corticosteroids.
When arthrocentesis is performed, the synovium may be inflamed, allowing fibrinogen, clotting factors, and other proteins to diffuse into the joint. Therefore, the collected synovial fluid should be placed in heparinized tubes to prevent clotting and to allow determination of cell type and cell number.
If diagnostic arthrocentesis is indicated, the aspirated joint fluid should be analyzed for volume, clarity, color, viscosity, cell count, culture, glucose, and protein. Synovial fluid is subsequently reported as normal, noninflammatory, inflammatory, or septic.15 Table 18-3 presents the characteristics of normal and three pathological types of synovial fluid. The presence and type of crystals in the fluid should be determined. The presence of crystals identified by polarized light microscopy with red compensation can be diagnostic (Table 18-4). (See Minicase 1.)
PMN = polymorphonuclear leukocyte; WBC = white blood cell.
CPPD = calcium pyrophosphate dihydrate; OA = osteoarthritis.
aThe property of birefringence is the ability of crystals to pass light in a particular plane. When viewed under polarized light, the crystals are brightly visible in one plane (birefringent), but are dark in a plane turned 90°. Birefringence observed under polarized light can be categorized as “positive” and “negative” based on the speed at which rays of light travel through the crystals in perpendicular planes (at right angles).
Assessment of Crystal-Induced Arthropathy
NORMAN S., AN 85-YEAR-OLD MAN, presented to the emergency department unable to bear weight on his right leg. Physical examination revealed a swollen, inflamed, and painful right knee without systemic signs or symptoms of infection. Norman S. was otherwise in good health except for several gout attacks over the past 10 years.
The right knee was aspirated and drained. Several drops of slightly cloudy, light yellow aspirate were placed on a slide and sent to pathology. The remaining aspirate was sent in heparinized tubes to the laboratory for Gram stain, bacterial culture, cell count, and chemistry panel.
Examination of the slide revealed a mixture of needle-shaped, rhomboid or rod, and variably shaped crystals. The slide was then viewed under a polarizing light microscope with a first-order red plate compensator. Most crystals demonstrated weak positive birefringence.
After receiving the pathology report, the emergency department physician reviewed the preliminary laboratory results of the knee aspirate (see Table 18-3 for reference values):
- 25,000 WBCs/mm3
- 55% PMNs
- 4.5 g/dL protein
- No bacteria or other organisms were seen on Gram stain
Question: What is the likely diagnosis in Norman S.? What additional laboratory studies should be performed?
Discussion: When Norman S. presented initially, septic arthritis would be high on the list of differential diagnoses. The absence of systemic signs and symptoms of infection does not rule out this condition. The aspiration of cloudy, yellow fluid from a red, swollen, and painful knee is consistent with infection and/or inflammation. Therefore, appropriate diagnostic tests were performed on the synovial fluid.
His history of gout may have suggested a recurrent acute gouty attack as the most likely diagnosis. However, microscopic examination revealed a mixture of crystal shapes, and polarizing light microscopy distinguished their most likely chemical composition. Based on the weak positive birefringence findings and variable crystal shapes, the crystals were probably composed of calcium pyrophosphate dihydrate (CPPD). Thus, the most likely diagnosis is calcium pyrophosphate deposition disease (pseudogout). In gouty arthritis, the monosodium urate crystals are needle-shaped and demonstrate bright negative birefringence. In both gout and pseudogout, phagocytosed crystals within polymorphonuclear leukocytes (PMNs) are usually observed in inflamed joints. The total synovial fluid leukocyte concentration is usually 15,000–30,000 cells/mm3, often with up to 90% neutrophils.
Additional laboratory studies that should be obtained include a serum uric acid level to rule out gout and SCr and BUN concentrations to assess kidney function. If the serum uric acid is elevated, consideration could be given to obtaining a 24-hour urine collection to determine if Norman S. is an overproducer or underexcretor of uric acid. These results could assist in the selection of prophylactic antihyperuricemic therapy, should that be considered desirable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree