Reversed Vein Bypass Grafts to Popliteal, Tibial, and Peroneal Arteries
Joseph L. Mills Sr.
Layla C. Lucas
The use of venous autografts dates back to the early 20th century and began with Alexis Carrel and Charles Guthrie’s description of the technique of vascular anastomosis in a canine model. In 1906, they published their experience of early bypass grafting using venous transplantation, the work for which Carrel was subsequently awarded the Nobel Prize (Physiology and Medicine) in 1912. In 1948, Jean Kunlin’s successful femoral-popliteal bypass using reversed saphenous vein graft in a patient of Rene Leriche generated enthusiasm for the arterial bypass era of vascular surgery, an era that lasted nearly half a century. Kunlin’s basic vein bypass technique remains in widespread use today. Although endovascular interventions are increasingly utilized to treat peripheral arterial disease (PAD), a significant number of patients continue to require open bypass either due to disease extent or after failure of endovascular therapy.
Patients with PAD generally present either with intermittent claudication (IC) or critical limb ischemia (CLI) manifested by rest pain, tissue loss, or gangrene. The diagnosis of PAD can usually be made based on a thorough history and physical examination. Claudicants most often complain of cramping or burning muscular calf, thigh, or buttock pain that occurs with walking, resolves with rest, and is reproduced by the same walking distance and exertion level. Ischemic rest pain is an early manifestation of CLI, typically occurring in the forefoot, with onset when the leg is flat or elevated and resolution when the limb is placed in a dependent position. The pain typically occurs when a patient goes to bed at night and is relieved by sleeping upright or hanging the affected limb over the edge of the bed. Patients with CLI may also develop non-healing ulcers that either arise spontaneously or in response to minor trauma. Gangrene is a late sign of CLI. Nearly universal physical findings in such patients include absent pedal pulses, trophic changes, and dependent rubor with pallor on elevation. Gangrene and non-healing ulcers typically appear on the toes and forefoot or over bony prominences, especially in those with diabetes. Patients with diabetes less commonly complain of rest pain; neuropathy is the underlying cause of most diabetic foot ulcers (DFU) that develop over bony prominences due to loss of protective sensation, deformity, and repetitive trauma. PAD is common in patients with DFU and contributes to non-healing or delayed healing in 30% to 50% of those so affected.
Non-invasive vascular laboratory tests are generally indicated in symptomatic PAD patients, especially when intervention is planned, to confirm the diagnosis, document the degree of limb ischemia, determine the anatomic site(s) of involvement, and differentiate stenotic lesions from totally occlusive lesions. The simplest baseline measurements are physiologic or hemodynamic tests. An ankle-brachial index (ABI) <0.9 is diagnostic for hemodynamically significant occlusive disease and has been found to be 95% sensitive in identifying angiographically confirmed PAD. Claudicants with single-level stenotic lesions may only show a reduced ABI after exercise. A 20% or greater reduction in the ABI post-exercise is abnormal. Arterial waveform patterns, toe waveforms and Doppler-derived pressures, and transcutaneous oxygen saturations are useful when the ABI cannot be measured due to medial calcinosis. Medial calcinosis is present when the ABI is >1.3 and is common in individuals with diabetes.
If intervention is planned, we generally next perform arterial duplex imaging. It is relatively inexpensive, does not require contrast or radiation exposure, is easily performed in the outpatient or office setting,
and readily identifies culprit lesions, sites of arterial occlusion, or stenosis, which are characterized by color flow changes, and elevated peak systolic velocities and velocity ratios. Duplex is extremely useful in predicting which patients are good candidates for endovascular therapy and aids in planning the best approach. We generally reserve computed tomography (CT) or magnetic resonance (MR) angiographic imaging for those patients who have markedly diminished or bilaterally absent femoral pulses. These imaging techniques define the length and extent of aortoiliac disease and the common femoral artery (CFA) disease burden, and also the factors critical in selecting whether endovascular therapy or major open surgery, such as aorto-bifemoral bypass, is the better option. We perform percutaneous angiography for those patients in whom endovascular therapy is planned, usually at the same setting, or for the patients with long segment femoro-popliteal and/or tibial disease in whom bypass is planned in order to identify optimal inflow and outflow sites.
and readily identifies culprit lesions, sites of arterial occlusion, or stenosis, which are characterized by color flow changes, and elevated peak systolic velocities and velocity ratios. Duplex is extremely useful in predicting which patients are good candidates for endovascular therapy and aids in planning the best approach. We generally reserve computed tomography (CT) or magnetic resonance (MR) angiographic imaging for those patients who have markedly diminished or bilaterally absent femoral pulses. These imaging techniques define the length and extent of aortoiliac disease and the common femoral artery (CFA) disease burden, and also the factors critical in selecting whether endovascular therapy or major open surgery, such as aorto-bifemoral bypass, is the better option. We perform percutaneous angiography for those patients in whom endovascular therapy is planned, usually at the same setting, or for the patients with long segment femoro-popliteal and/or tibial disease in whom bypass is planned in order to identify optimal inflow and outflow sites.
PAD is a systemic disease and a coronary artery disease (CAD) “equivalent.” PAD is more of a marker for cardiovascular mortality, heart attack, and stroke than it is a harbinger of limb loss. Mortality in patients with PAD averages 2% per year, and the incidence of non-fatal myocardial infarction, stroke, and vascular death is estimated at 5% to 7% per year for PAD patients. Therefore, medical therapy and risk factor modification are mandatory in all PAD patients, regardless of whether intervention is planned. Treating lesions by endovascular therapy or open bypass surgery without maximizing these noninvasive aspects of care is inadequate therapy.
The Trans-Atlantic Inter-Society Consensus Document (TASC II) and the Society for Vascular Surgery (SVS) and American Heart Association (AHA) guidelines all offer evidence-based recommendations for the medical management of important PAD risk factors. The major modifiable risk factors are smoking, hyperlipidemia, hypertension, and diabetes. Antiplatelet therapy should be routinely administered.
Smoking is an independent PAD risk factor for PAD, increasing its prevalence and severity. Smokers are three to five times more likely to require amputation than non-smokers. Physician advice, group counseling, nicotine replacement, and antidepressant drug therapy increase the success of tobacco abstinence.
Hyperlipidemia is an independent risk factor for PAD best controlled by statin administration and dietary modification. Target levels are a low-density lipoprotein (LDL) <70 mg/dL in patients with coronary disease/PAD and <100 mg/dL in those without. Fibrates and/or niacin are useful in lowering triglycerides and in raising HDL (which has a protective effect).
Hypertension is another major PAD risk factor. Blood pressure control can reduce PAD-related complications by 22% to 26% and also significantly reduces both cardiovascular and cerebrovascular events. Current recommendations are a target blood pressure <140/90 mm Hg (<130/80 mm Hg in those with diabetes or renal insufficiency) in PAD patients. Comprehensive diabetes management is also an important component of PAD care. Large-scale studies have shown that diabetes management with a target hemoglobin A1c <7.0% reduces diabetes-related myocardial infarcts and other diabetes-related endpoints.
Antiplatelet therapy is an essential tool to reduce subsequent cardiovascular and cerebrovascular events. Daily aspirin therapy confers a 25% reduction in cardiovascular events. Low-dose aspirin is as effective as full-dose aspirin with fewer adverse reactions. Other antiplatelet drugs are effective but carry a higher risk of bleeding.
The presence of either CLI or lifestyle-limiting IC is an indication for intervention. Once the diagnosis is established and the extent of disease determined, an assessment of the patient’s ability to tolerate a major revascularization is necessary. A chest radiograph and electrocardiogram will give rough estimates of cardiopulmonary status. Further cardiac evaluation and/or optimization are recommended if the patient has unstable angina, significant arrhythmias, or symptoms of congestive heart failure.
Preoperative Planning
Selection of Inflow Artery
The inflow target for femoral-popliteal bypass is based on physical examination and non-invasive studies and confirmed by preoperative or immediate pre-bypass angiography. Hemodynamically significant aorto-iliac inflow lesions should be addressed before or in conjunction with the infrainguinal bypass procedure. Although the CFA frequently serves as the graft origin, distal origin sites, when appropriately selected, are equally effective, especially in redo operations and when vein conduit is limited. If the CFA is significantly diseased, it is prudent to consider CFA endarterectomy to include the profunda femoris artery (PFA) origin if also involved. CFA exposure may be prohibitive in reoperative procedures or morbidly obese patients. The PFA or superficial femoral artery (SFA) can often serve as alternate inflow sources. Regardless of site of origin, adequate inflow is crucial to bypass success.
Selection of Outflow (Target) Artery
The distal target artery is ideally of normal caliber, is free of stenosis and, in general, should have continuity with at least one of the arteries supplying the foot. The above-knee popliteal (AKP) artery has a higher incidence of atherosclerotic disease extending from the SFA. Therefore, the below-knee popliteal (BKP) artery is more commonly used for femoral-popliteal bypass. If the popliteal artery (PA) is very diseased or occluded, the tibial artery with the best runoff to the foot should be chosen as the target. In selected cases, it is important to consider the location of the foot ulcer. In particular, the angiosome model may be important to consider in patients with extensive pedal and arch occlusive disease in whom hindfoot and forefoot flow may be compartmentalized. A common example of this issue includes deep heel ulcers in patients with renal failure. Improper selection of the outflow target artery may precipitate graft failure and potentially jeopardize limb viability. Both preoperative duplex imaging and detailed angiography, including lateral magnified views of the foot in CLI patients, are essential in selecting the most appropriate target.
Preoperative Assessment of Vein Quality
A complete history and physical examination will often reveal whether the great saphenous vein (GSV) has been used for coronary bypass, previous lower extremity revascularization, or stripped for varicosities. Preoperative venous duplex mapping should be done if there is any question about the adequacy of the vein conduit. Vein mapping should evaluate the diameter, compressibility, wall thickness, and flow through the proposed conduit, including examination of the cephalic, basilic, and small saphenous veins if the GSV is absent or inadequate. Vein conduit diameter should be at least 3 mm to optimize patency. The location of the vein should be marked on the skin with an indelible marker to facilitate harvest and minimize creation of skin flaps during harvest.
Conduit Selection
Autogenous vein grafts provide the best patency for use in arterial bypass for all infrainguinal reconstructions, regardless of distal target. The conduit of choice for a femoral-popliteal bypass is therefore ipsilateral GSV. Reversed and in situ configurations are equally effective, but reversed vein is applicable to more patients and generally simpler to employ. Although the Arizona group and others prefer reversed vein, some other groups have achieved equivalent success with in situ vein conduits. If ipsilateral GSV is inadequate or unavailable, contralateral GSV is the next best option. Splicing the short saphenous veins (SSVs), and/or the cephalic and basilic veins from the arms often provides enough length for bypass in the absence of suitable leg vein.
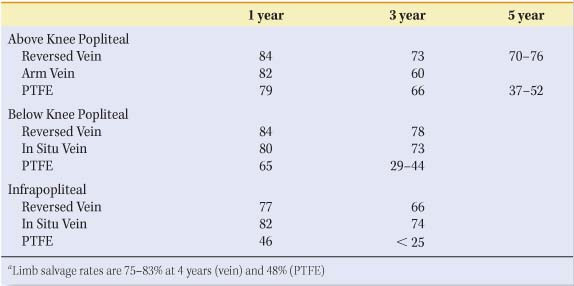
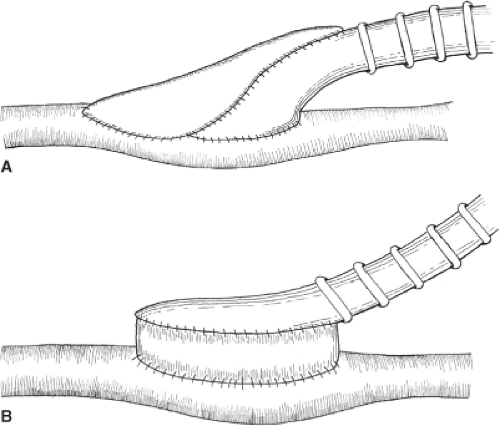
Non-autogenous options exist for the uncommon patient who truly lacks suitable vein conduit. The most commonly used prosthetic conduit for femoral-popliteal bypass is polytetrafluoroethylene (PTFE). Varieties of PTFE grafts may be coated with heparin or carbon-impregnated, but their long-term patency is similar to untreated PTFE grafts. In femoral AKP bypass, PTFE grafts have the same patency as RSVG for the first 2 years, but vein is superior thereafter and is therefore preferable. Prosthetic bypasses to the below-knee popliteal and tibial arteries have uniformly poor patency that can be modestly improved by the use of an adjunctive vein cuff (Miller or St. Mary’s) or Taylor patch (Fig. 1). Cryopreserved vein grafts have exceedingly poor patency rates and should be reserved for limb salvage indications; they may also have a niche in the management of patients with infected prosthetic grafts.
Table 1 Expected Primary Patency Rates (%) of Infrainguinal Bypass Grafts | |
|---|---|
|
in Situ or Rsvg
Nearly all randomized trials have shown equivalent patency and limb salvage rates for in situ and reversed vein. Small-caliber veins perform poorly in either configuration. Reversed vein conduits are user-friendly and more readily adaptable to a variety of clinical scenarios. Expected graft patency rates are shown in Table 1.
Surgical Anatomy of the Femoral, Popliteal, and Tibial Arteries
The femoral triangle is an important anatomic space bordered by the inguinal ligament superiorly, the sartorius muscle laterally, and the adductor longus muscle medially. The floor of the femoral triangle consists of the iliacus, psoas major, pectineus, and adductor longus muscles. A fascial covering, the femoral sheath, contains the major vascular structures in the femoral triangle. The CFA is the continuation of the external iliac artery below the inguinal ligament. In the femoral triangle, it divides into two major branches (Fig. 2). The PFA is most commonly a posterolateral branch and not only provides blood to the thigh, but also serves as an important collateral in patients with SFA-occlusive disease. The SFA is the continuation of the CFA and supplies blood to the lower leg. Exiting the femoral triangle, the SFA proceeds distally on its course into the adductor canal. The common femoral vein (CFV) is located just medially to the CFA in the triangle. The GSV enters the CFV at the fossa ovalis. Other important structures in the femoral triangle include the femoral nerve, lying lateral to the artery and providing motor and sensory function to the lower extremity. The lymphatics are medial to the CFV.
The popliteal fossa is another important anatomic location in lower extremity revascularization. It is a diamond-shaped space defined anteriorly by the femur, upper tibia, and popliteus muscle; posteriorly by the skin, subcutaneous tissue, and fascia; laterally by biceps femoris and gastrocnemius muscles; medially by semitendinosus and semimembranosus muscles. The SFA exits the adductor canal at the apex of the popliteal fossa where it becomes the PA. Paired popliteal veins are closely adjacent.
The below-knee PA is of variable length in the distal popliteal fossa before it divides into the anterior tibial (AT) artery and the tibioperoneal (TP) trunk. The AT exits laterally and enters the anterior compartment
of the lower leg. The TP continues and branches into the posterior tibial (PT) artery and the peroneal artery, which enter the deep posterior compartment of the lower leg. They are exposed in their proximal and mid-portions through medial calf incisions, usually through the same incision that is used to harvest the GSV. After dividing the fascia to enter the superficial posterior compartment, the medial head of the gastrocnemius muscle is retracted posteriorly. The soleus is taken down off the tibia to enter the deep posterior compartment, within which the PT artery is more superficially located, whereas the peroneal artery is deeper, adjacent to the fibula.
of the lower leg. The TP continues and branches into the posterior tibial (PT) artery and the peroneal artery, which enter the deep posterior compartment of the lower leg. They are exposed in their proximal and mid-portions through medial calf incisions, usually through the same incision that is used to harvest the GSV. After dividing the fascia to enter the superficial posterior compartment, the medial head of the gastrocnemius muscle is retracted posteriorly. The soleus is taken down off the tibia to enter the deep posterior compartment, within which the PT artery is more superficially located, whereas the peroneal artery is deeper, adjacent to the fibula.
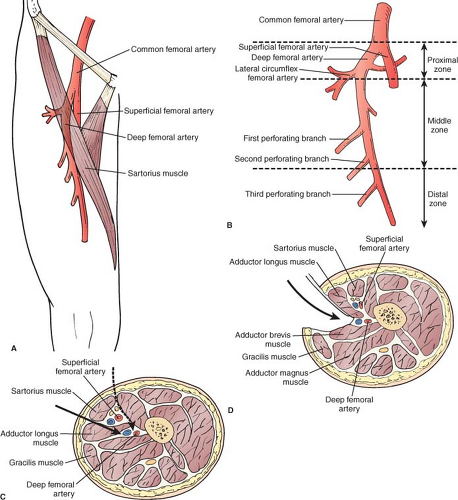 Fig. 2. Alternate approaches to the distal deep femoral artery (DFA) are useful for reoperative femoral-distal bypass cases and when it is desirable to shorten the bypass due to limited length of vein conduit. A: Anatomy of the DFA showing surface landmarks used to identify the course of the DFA. B: The DFA can be divided into three zones. C: Transverse section of the thigh (viewed from above) shows the plane of dissection when the anterior-medial approach (solid arrow) is used. Alternatively, the DFA can be approached through an even more anterior route (dashed arrow) by an incision along the lateral border of the sartorius and by retracting this muscle and the superficial femoral neurovascular bundle medially to reach the distal DFA. D: Posterior approach to the distal DFA. Note the fascial plane and structures separating DFA from superficial femoral vascular structures and isolating it from the subsartorial canal. (From: Rutherford’s Vascular Surgery, 7th Edition, Saunders Elsevier, 2010, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|