Retropubic, Laparoscopic, and Robotic-Assisted Radical Prostatectomy
Andrew A. Wagner
Martin G. Sanda
Evolution of Radical Prostatectomy
Radical retropubic prostatectomy gained widespread acceptance for early stage prostate cancer after Walsh and Donker discovered nuances of periprostatic anatomy that led to reduced blood loss and improved functional recovery. However, the observation that early stage prostate cancer poses only limited risk to long-term mortality has fueled skepticism as to whether radical surgical intervention is justifiable. This dilemma was recently addressed, when a multi-institutional phase III trial showed overall survival benefit among patients randomized to retropubic radical prostatectomy (RRP) over those randomized to watchful waiting. The survival advantage was particularly evident in men younger than 65 years. Therefore, for men who are suitable candidates for surgical intervention, retropubic prostatectomy is the standard of care by which other surgical or nonsurgical interventions for early stage prostate cancer can be measured.
Surgeons interested in further refining radical prostatectomy examined minimally invasive approaches in the 1990s. Schuessler and colleagues described the first limited series of nine laparoscopic radical prostatectomy cases in 1997; however, they quickly abandoned the approach due to long operative times and hospital stays. This straight laparoscopic approach was not adopted into urology until refinements in task-specific instrumentation and laparoscopic technique led two centers in France to standardize the procedure in 2000, allowing for laparoscopic prostatectomy to be safe and reproducible. Despite these successes, straight laparoscopic prostatectomy was very difficult to learn and perform consistently. The introduction of robotic-assisted laparoscopic surgery using the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA) has allowed some of the more technically complex aspects of the procedure to be more easily and consistently reproduced by surgeons, even those without advanced laparoscopic training, thus allowing more patients access the benefits of minimally invasive surgery. The robotic platform is especially useful for reconstructive procedures in confined spaces, for example, the male pelvis. Widespread adoption of robotic technology and direct-to-patient marketing has changed prostate cancer care such that currently the robotic approach is the most common approach for radical prostatectomy. Although there are widely agreed-upon benefits to the robotic approach with regard to improvements in
anatomic visualization, refined instrument control, less blood loss, and improved surgical cosmesis, cancer control and functional results after prostatectomy are more a function of surgeon skill, experience, and training rather than approach (robotic vs. open).
anatomic visualization, refined instrument control, less blood loss, and improved surgical cosmesis, cancer control and functional results after prostatectomy are more a function of surgeon skill, experience, and training rather than approach (robotic vs. open).
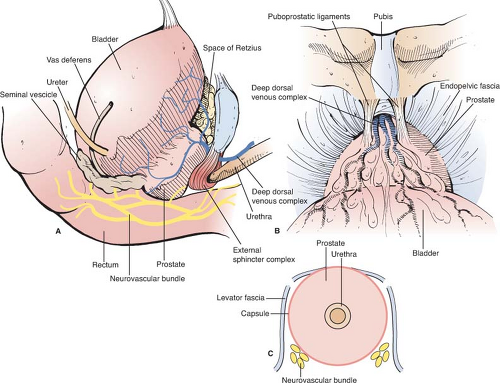
The prostate is a male secretory gland located in the retropubic space (Fig. 1). As men age, it tends to increase in overall size and typically measures 20 to 60 g in middle adulthood. It is composed of a central zone (also called transitional zone) and a peripheral zone. The majority of prostate adenocarcinoma is located in the peripheral zone and the posterior aspect of this zone can be palpated by performing a digital rectal exam. The urethra passes through the central zone on its way out to the membranous and penile sections of urethra. A sling-like sheath of striated muscle, known as the external urethral sphincter, surrounds the membranous section of urethra. Once the smooth muscle of the prostate and bladder neck are violated during prostatectomy, it is the external urethral sphincter that largely determines the speed and level of urinary functional recovery, that is, continence that a patient recovers. Posterolateral to the prostate are two neurovascular bundles (NVBs), which lie in direct apposition to the prostatic capsule and course to the corpora cavernosa posterolateral to the urethra (Fig. 1C). These bundles can be peeled off the surface of the prostate in appropriate candidates (i.e., those without high grade or high volume cancer), thus allowing those patients an opportunity to regain erectile function after surgery. The rectum lies directly posterior to the prostate, the two organs being separated by two leaves of Denonvillier’s fascia.
The majority of prostate cancer is detected in asymptomatic men through routine screening with prostate-specific antigen and digital rectal examination, and 85% of prostate cancer is clinically localized at the time of diagnosis. Although these tests have vastly improved early detection of prostate cancer, neither test is particularly sensitive or specific. Combining the tests improves detection accuracy over either test alone and therefore when performed in informed patients, they allow for men with early stage disease to be diagnosed and treated before local symptoms or metastasis occur. When
the level of suspicion is raised based on PSA and DRE (taking into account other factors such as age, race, and family history), a transrectal, ultrasound-guided prostate biopsy can be performed. This is a 5-minute office procedure using transrectal ultrasound to guide 12–20 core needle biopsies after performing a periprostatic block with local anesthetic. The biopsy is generally well tolerated; however, there is a 1% risk of bleeding or bacteremia afterward.
the level of suspicion is raised based on PSA and DRE (taking into account other factors such as age, race, and family history), a transrectal, ultrasound-guided prostate biopsy can be performed. This is a 5-minute office procedure using transrectal ultrasound to guide 12–20 core needle biopsies after performing a periprostatic block with local anesthetic. The biopsy is generally well tolerated; however, there is a 1% risk of bleeding or bacteremia afterward.
Management Options for Localized Prostate Cancer: Deciding Whom and How to Treat
Patients with early stage prostate cancer face several care options, including active surveillance, prostatectomy, brachytherapy, or various forms of external radiotherapy. The principal consideration in deciding between these care options is the aggressiveness of the primary tumor, which is defined based on parameters including histological grade of the cancer (measured by the Gleason score), clinical stage (determined by digital rectal examination), and serum PSA level. Based on these three parameters, aggressiveness of the cancer can then be categorized as low-risk, intermediate-risk, and high-risk tumors (Table 1).
Table 1 Risk and Benefit of Various Approaches to Managing Early Stage Prostate Cancer | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Prostatectomy is a suitable care option for all three risk categories; however, class I evidence supporting benefit from prostatectomy has been reported only for intermediate- and high-risk disease. External radiotherapy has also shown survival benefit for intermediate- and high-risk disease; however, class I evidence of such survival benefit from radiotherapy has been limited to settings wherein radiotherapy is combined with neoadjuvant hormonal therapy. Brachytherapy is commonly used to manage low-risk cancers based on several multicenter studies showing elimination of primary tumors by brachytherapy, but class I evidence of a survival benefit from brachytherapy is lacking.
Each of the primary treatment options for early stage prostate cancer (prostatectomy, external radiotherapy, and brachytherapy) has associated side effects that can compromise long-term quality of life. Erectile dysfunction is the most common long-term quality of life consequence after early stage prostate cancer treatment, affecting more than half of treated patients. Urinary problems (incontinence after prostatectomy vs. obstructive or irritative urinary problems after radiotherapy or brachytherapy) become a moderate or severe long-term problem for about 1 in 10 treated men, while proctitis affects 1 in 10 men long-term after radiotherapy and 1 in 20 after brachytherapy.
The pervasiveness of treatment side effects despite contemporary refinements in surgical technique (e.g., laparoscopy and robotics) or radiotherapy (e.g., use of intensity-modulated radiotherapy, proton beam therapy, or hyper-fractionation techniques) has led to renewed interest in active surveillance as an alternative to immediate treatment for low-risk prostate cancers. Active surveillance entails attentive monitoring, via PSA testing, imaging, and repeat biopsy at regular intervals to evaluate for tumor progression. Recent work suggests that intervention after initial active surveillance is as effective as immediate intervention, while avoiding treatment in half of men with low-risk cancer.
For many men, a decision as to which care option is appropriate can be facilitated by consideration of related health concerns at time of diagnosis. For men who have preexisting erectile dysfunction (as seen in one-third of patients with newly diagnosed prostate cancer), the detriment of each treatment is substantially mitigated, weighing in favor of intervention. For men who have preexisting obstructive urinary symptoms at diagnosis (one-fifth of newly diagnosed prostate cancer patients), prostatectomy is preferable as it can actually improve quality of life in this setting. For some patients, contraindications to prostatectomy or radiotherapy can facilitate choosing a care option. However,
many men and their physicians are left with the difficult task of selecting a care options in the setting of abundant flawed studies yet sparse class I evidence.
many men and their physicians are left with the difficult task of selecting a care options in the setting of abundant flawed studies yet sparse class I evidence.
The standard indication for RRP or robotic-assisted prostatectomy (RAP) is prostate cancer confined to the vicinity of the prostate (clinical stage ≤T2) without evidence of metastasis. Contraindications include coagulopathy, thrombocytopenia, or presence of cardiopulmonary or other comorbidities that would weigh against surgical therapy, in consideration of other available prostate cancer therapy such as radiotherapy or hormonal therapy.
Complicated scenarios such as prior pelvic radiation, neoadjuvant hormonal or chemotherapy, morbid obesity, large prostate size (e.g., >100 g), or prior abdominal or prostate surgery (e.g., transurethral resection of the prostate) can be expected to compound the technical challenges of prostatectomy, either robotic or open. However, either approach can be used depending on the experience of the surgeon.
Preoperative patient preparation prior to standard retropubic prostatectomy can be limited to a fleet enema on the evening prior to surgery, and administration of preoperative antibiotics prior to surgical incision. Alternatively, 300 cc of magnesium citrate can be used as a bowel preparation on the day prior to surgery. Depending on preference of the surgeon and anesthesiologist, RRP can be performed either under general anesthetic or with use of regional anesthesia (epidural or spinal) with concurrent intravenous sedation. Reduced blood loss has been associated with use of epidural anesthesia without the use of concurrent general anesthetic.
Patient Positioning, Incision, and Exposing the Preperitoneal Space of Retzius
Sequential compression devices are placed prior to induction. The patient is positioned supine with moderate hyperextension at the level of the umbilicus. The sterile field is established to include the abdomen from the umbilicus to below the penis. A Foley catheter with a 30-cc balloon (to facilitate bladder retraction) is placed. A 12- to 15-cm midline incision is made from above the pubic symphysis to below the umbilicus. The abdominal fascia is incised in the midline, and rectus abdominus muscles are retracted laterally to expose the preperitoneal space of Retzius (Fig. 1). Either fixed retraction (Bookwalter retractor) or Balfour retractor are used for lateral retraction of the rectus abdominus muscles. Blunt dissection exposes the iliac vessels, the vasa deferentia, obturator fossae, and endopelvic fascia bilaterally.
Pelvic Lymphadenectomy
Obturator and external iliac lymph nodes can be removed for staging purposes. Although historically performed routinely during radical prostatectomy, lymphadenectomy can also be performed selectively in just those cases with risk factors for extraprostatic extension (such as serum PSA >10 ng/mL or Gleason score >6). The purpose of the lymphadenectomy in radical prostatectomy is purely diagnostic, with the results possibly influencing use of adjuvant therapies or frequency of follow-up. For pelvic lymphadenectomy, borders include the external iliac vein anteriorly, the obturator nerve posteriorly, the bifurcation of the iliac vein as the cephalad extent, and the pelvic side wall as the caudal extent of the removed lymph node specimen. One or two clips placed at the proximal and distal border of the nodal packet are usually sufficient to prevent significant lymphatic leak.
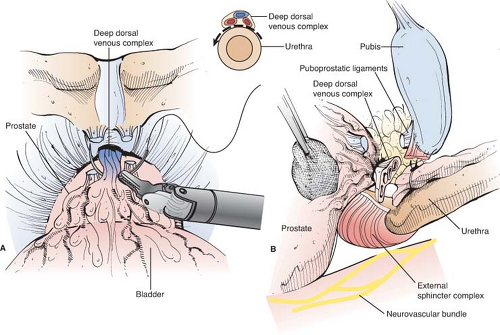
The Deep Dorsal Vein and Anterior Prostatic Apex
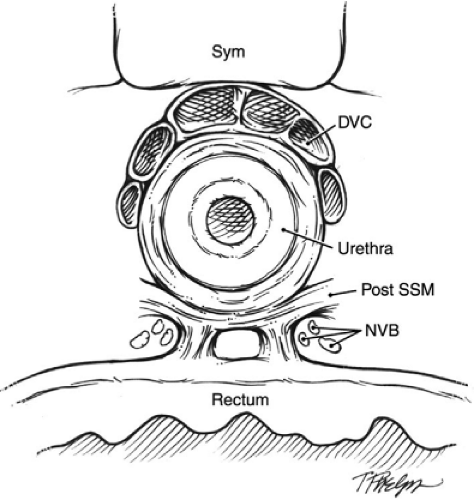
Many components of the dissection of the prostate, nerve-sparing, and subsequent vesicourethral anastamosis are performed using modifications of technique first described by Walsh. To expose the deep dorsal vein and anterior prostatic apex (Fig. 1B), a notched retractor blade is used to retract the bladder superiorly by traction on the Foley balloon catheter within the bladder dome. The endopelvic fascia is incised, puboprostatic ligaments are divided with scissors, and superficial dorsal vein is cauterized and divided. Excessive use of electrocautery beyond the prostatic apex should be avoided, as such can injure sphincteric nerves. Cephalad retraction of the prostatic apex by a sponge-stick improves retropubic exposure of the deep dorsal vein. For hemostasis before dividing the deep dorsal vein, two figure-of-eight suture ligatures (0-chromic on a CT-1 needle) are placed to secure the deep dorsal vein proximally and distally (Fig. 2). These suture ligatures can control the anterior component of the dorsal venous complex (DVC), but in many cases may not control the posterolateral extensions of the deep dorsal vein. A harmonic scalpel or ultrasonic shears are advantageous for division of the deep DVC as these provide improved hemostasis during division of the dorsal vein, particularly in the posterolateral extension of this venous complex as it drapes around the membranous urethra. Care again should be taken to avoid excessive use of ultrasonic or harmonic scissors as these can generate significant heat that can cause thermal injury to adjacent nerves. The cavernous nerves and sphincteric nerves course posterolateral to the membranous urethra (Fig. 3), so use of the harmonic scalpel posterior to the urethra should be avoided. In cases when hemostasis is not adequate following division of the deep dorsal vein, the distal venous complex can be oversewn with a running 2-0 chromic suture on a UR-6 needle.
Dissecting the Membranous Urethra and Prostatic Apex
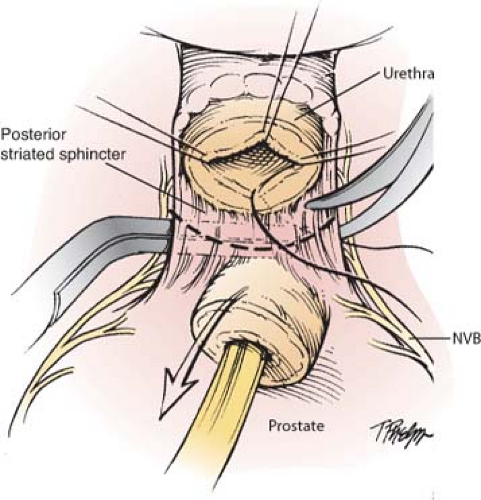
Division of the deep dorsal vein exposes the urethra below. After incision of the anterior and lateral urethral wall with scissors, sutures for the eventual vesicourethral anastomosis (2-0 monocryl) are anchored in the urethra at 12 o’clock, 3 o’clock, and 9 o’clock positions. The Foley catheter is then divided at the urethral meatus, the cut end withdrawn into the pelvis and there secured with a cocker clamp to keep the balloon inflated in the bladder, to later serve for traction and as a guide for bladder neck transection. The fourth anastomotic suture is placed at 6 o’clock, and the posterior urethra is then cut to complete transection of the urethra at the prostatic apex. The posterior striated sphincter (rectourethralis muscle) is then divided sharply to reveal Denonvillier’s fascia beyond the edge of the posterior prostatic apex below (Fig. 4).
Posterolateral Dissection of the Cavernous Nerves and Prostatic Pedicles
Denonvillier’s fascia is divided sharply in the midline, beyond the prostatic apex and between the laterally positioned NVBs (comprised of cavernous nerves that mediate erectile function and sphincteric nerves
that mediate urethral sphincter tone). For nerve-sparing dissection, the lateral pelvic fascia (that covers the lateral prostate and cavernous NVB) is elevated from the prostate and nerves below with a sharp right angle clamp and is divided sharply from prostatic apex to the bladder neck. This maneuver exposes the prostatic border and NVB, and also releases the NVB laterally, facilitating its subsequent preservation (Fig. 5). A fine-tipped right angle clamp is then used to sequentially separate the remaining attachments of the NVB from the lateral border of the prostate, with vascular branches clipped for hemostasis while minimizing traumatic traction to the NVB by minimizing cephalad traction on the prostate. During the retrograde dissection (Fig. 6), the mid-posterior prostate capsule and Denonvillier’s fascia are separated from the prerectal fat in the midline, although fibrous remnants from prostate biopsies can be encountered, and these are best divided sharply with scissors. At the level of the seminal vesicles, Denonvilier’s fascia is again incised sharply, and posterolateral vascular pedicles to the prostate are
clipped and divided to reveal the border between the seminal vesicle and posterior bladder neck. The space between the seminal vesicle and bladder neck is developed with a blunt right angle clamp that can be passed entirely though the space from right to left–this maneuver, mobilizing the seminal vesicles away from the posterior bladder neck, facilitates subsequent transection of the posterior bladder neck.
that mediate urethral sphincter tone). For nerve-sparing dissection, the lateral pelvic fascia (that covers the lateral prostate and cavernous NVB) is elevated from the prostate and nerves below with a sharp right angle clamp and is divided sharply from prostatic apex to the bladder neck. This maneuver exposes the prostatic border and NVB, and also releases the NVB laterally, facilitating its subsequent preservation (Fig. 5). A fine-tipped right angle clamp is then used to sequentially separate the remaining attachments of the NVB from the lateral border of the prostate, with vascular branches clipped for hemostasis while minimizing traumatic traction to the NVB by minimizing cephalad traction on the prostate. During the retrograde dissection (Fig. 6), the mid-posterior prostate capsule and Denonvillier’s fascia are separated from the prerectal fat in the midline, although fibrous remnants from prostate biopsies can be encountered, and these are best divided sharply with scissors. At the level of the seminal vesicles, Denonvilier’s fascia is again incised sharply, and posterolateral vascular pedicles to the prostate are
clipped and divided to reveal the border between the seminal vesicle and posterior bladder neck. The space between the seminal vesicle and bladder neck is developed with a blunt right angle clamp that can be passed entirely though the space from right to left–this maneuver, mobilizing the seminal vesicles away from the posterior bladder neck, facilitates subsequent transection of the posterior bladder neck.
Wide Excision of the Neurovascular Bundle
If preoperative studies (e.g., findings on rectal examination or endorectal coil, contrast-enhanced MRI) or intraoperative findings indicate presence of tumor close enough to the NVB that sparing of the cavernous nerves would jeopardize surgical margins, then the NVB on the affected side should be widely excised. Wide excision is accomplished by retrograde dissection identical to that described above, except that the lateral pelvic fascia is not incised, and hence the NVB and any associated extraprostatic tumor remains closely attached to the posterolateral border of the prostate. The dissection proceeds laterally across the NVB beyond the prostatic apex, clipping and dividing the bundle immediately after it is exposed following division of the rectourethralis muscle and incision of Denonvillier’s fascia beyond the prostatic apex. The retrograde dissection separating the prostate from rectum proceeds superiorly and laterally beyond the NVB, using clips for hemostasis when necessary. When the posterolateral pedicles to the prostate are encountered they are managed as described above.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree