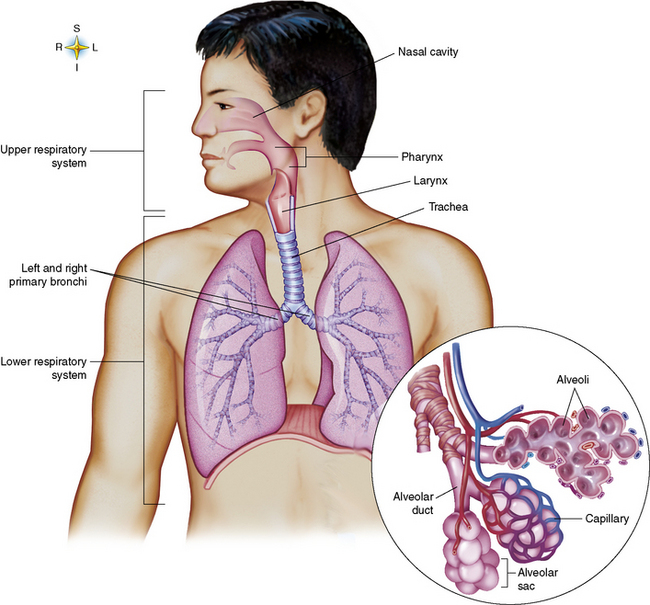
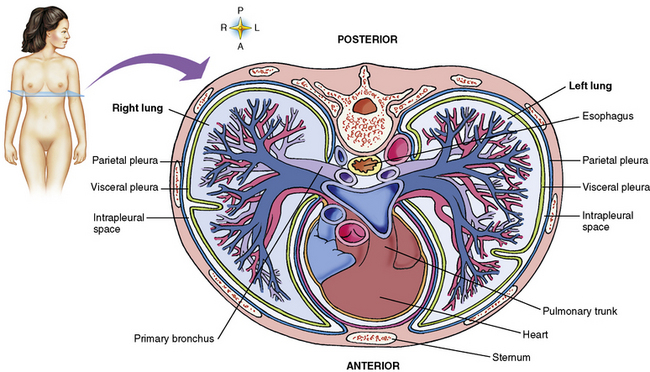
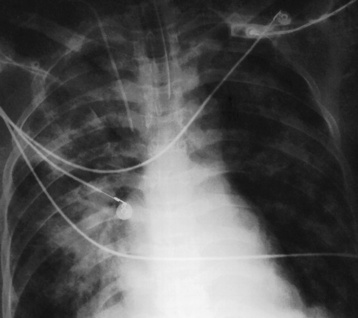
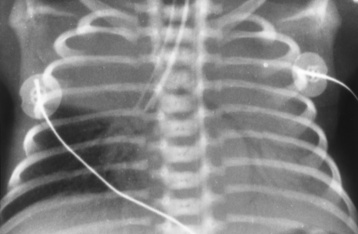
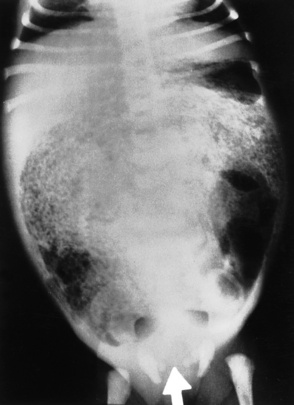
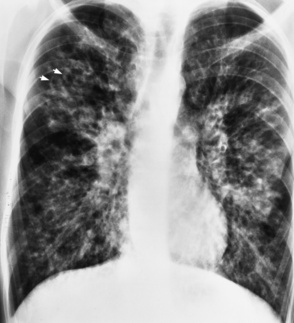
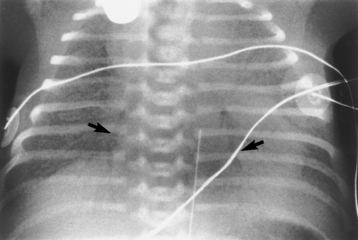
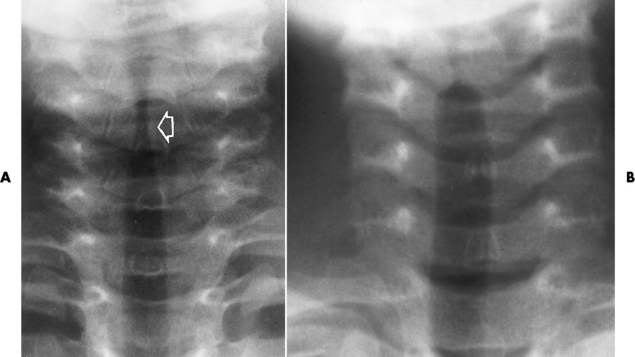
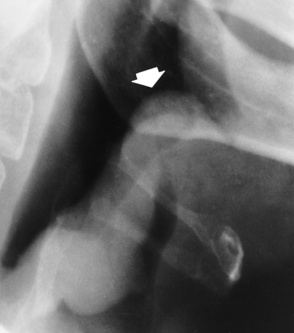
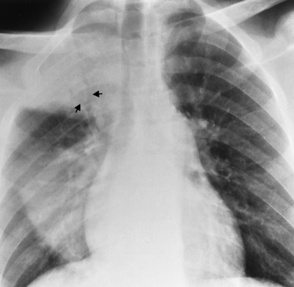
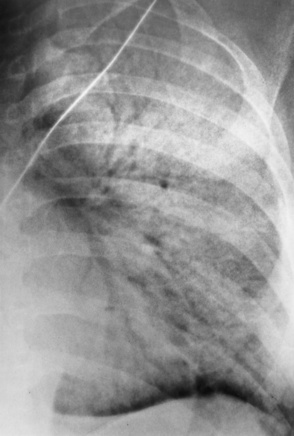
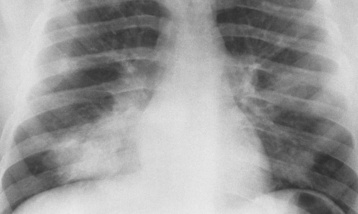
Chapter 3 Physiology of the Respiratory System Congenital/Hereditary Diseases Inflammatory Disorders of the Upper Respiratory System Inflammatory Disorders of the Lower Respiratory System After reading this chapter, the reader will be able to: 1 Locate placement for an endotracheal tube, central venous catheter, Swan-Ganz catheter, and transvenous cardiac pacemaker 2 Recognize the most common complications involved with improper placement of these tubes and catheters and how chest radiography plays an important role in diagnosing them 3 Classify the more common diseases in terms of their attenuation of x-rays 4 Explain the changes in technical factors required to obtain optimal quality radiographs for patients with various underlying pathologic conditions 5 Define and describe all bold-faced terms in this chapter 6 Describe the physiology of the respiratory system 7 Identify anatomic structures on both diagrams and radiographs of the respiratory system 8 Differentiate the more common pathologic conditions affecting the respiratory system and their radiographic manifestations The major role of the respiratory system is the oxygenation of blood and the removal of the body’s waste products in the form of carbon dioxide. The respiratory system consists of two separate divisions, the upper tract located outside the thorax and lower tract found within the thoracic cavity (Figure 3-1). The upper respiratory system, which consists of the nasopharynx, oropharynx, and larynx, provides structure for the passage of air into the lower respiratory system. The lower respiratory system, which consists of the trachea, bronchi, and bronchioles, is composed of tubular structures responsible for conducting air from the upper respiratory structures. The smallest unit where gas exchange occurs consists of the terminal bronchiole, alveolar ducts, and alveolar sacs. With the use of the upper and lower respiratory structures, the air from outside the body enters the lungs. The single trachea branches out into two bronchi (one to each lung) at the carina (last segment of the trachea), which in turn branch out into progressively smaller bronchioles to produce a structure termed the bronchial tree because its appearance resembles an inverted tree. The tracheobronchial tree is lined with a mucous membrane (the respiratory epithelium) containing numerous hairlike projections called cilia. During inspiration, the air is moistened and warmed as it enters the lungs. The cilia act as miniature sweepers to prevent dust and foreign particles from reaching the lungs. When the ciliary blanket works correctly, the particles are moved away from the lungs to be coughed up or swallowed. Any damage to the respiratory epithelium and its cilia permits particles (entering with the inspired or inhaled air) to proliferate and produce a disease process. The vital gas exchange within the lung (called external respiration) takes place within the alveoli, extremely thin-walled sacs surrounded by blood capillaries, which represent the true parenchyma of the lung (see Figure 3-1 inset). Oxygen in the inhaled air diffuses from the alveoli into the blood capillaries, where it attaches to hemoglobin molecules in red blood cells and circulates to the various tissues of the body (called internal respiration). Carbon dioxide, a waste product of cellular metabolism, diffuses in the opposite direction, passing from the blood capillaries into the alveoli and then exiting the body during expiration (or exhalation). Because individual alveoli are extremely small, chest radiographs can demonstrate only a cluster of alveoli and their tiny terminal bronchioles, which are the basic anatomic units of the lung. A cluster of alveoli is termed the acinus. A double-walled membrane consisting of two layers of pleura encases the lungs (Figure 3-2). The visceral pleura is the inner layer that adheres to the lung, whereas the parietal pleura lines the inner chest wall (the thoracic cavity). Between the two layers of pleura is a potential space (pleural space), which normally contains only a small amount of fluid to lubricate the surfaces to prevent friction as the lungs expand and contract. The airtight space between the lungs and the chest wall has a pressure a bit less than that in the lungs. This difference in pressure acts like a vacuum to prevent the lungs from collapsing. An inflammatory or neoplastic process that involves the pleura may produce fluid within the potential space (a pleural effusion). The relationship between the tip of the tube and the carina (tracheal bifurcation) must be carefully assessed. When the head and neck are in a neutral position, the endotracheal tube tip ideally should be about 5 to 7 cm above the carina (Figure 3-3). With flexion and extension of the neck, the tip of the tube will move about 2 cm caudally and cranially, respectively. About 10% to 20% of endotracheal tubes require repositioning after insertion. A tube positioned too low usually extends into the right mainstem bronchus, where it eventually leads to atelectasis of the left lung (see Figure 3-76). A tube positioned excessively high or in the esophagus causes the inspired air to enter the stomach, causing severe gastric dilatation and a high likelihood of regurgitation of gastric contents and aspiration pneumonia. Because up to one third of CVP catheters are initially inserted incorrectly, the position of the catheter should be confirmed by a chest radiograph. The most common aberrant location of a CVP catheter is the internal jugular vein (Figure 3-4). CVP catheters that extend to the right atrium are associated with an increased risk of cardiac arrhythmias and even perforation. Extension of the catheter into the hepatic veins may result in the infusion of potentially toxic substances (some antibiotics and hypertonic alimentation solutions) directly into the liver. Even after successful placement, CVP catheters may change position as a result of patient motion or medical manipulation. Therefore, periodic radiographic confirmation of the catheter position is often recommended. The anatomy of the subclavian region may lead to complications when a central catheter is introduced via the subclavian vein. Because the pleura covering the apex of the lung lies just deep to the subclavian vein, a pneumothorax may develop. This problem may be difficult to detect clinically, and thus a chest radiograph (if possible with the patient in an upright position and in expiration) should be obtained whenever insertion of a subclavian catheter has been attempted. Another complication is perivascular CVP catheter placement, which may result in ectopic infusion of fluid into the mediastinum or pleural space. This diagnosis should be suggested if there is rapid development of mediastinal widening or pleural effusion after CVP catheter insertion (Figure 3-5). Other complications include inadvertent puncture of the subclavian artery, air embolism, and injury to the phrenic nerve. The peripherally inserted central catheter (PICC) has become the long-term venous access device used for home therapy and for patients undergoing chemotherapy (Figure 3-6). Catheter breakage and embolization can result from laceration of the catheter by the needle used to insert it, fracture at a point of stress, or detachment of the catheter from its hub. The catheter fragment may lodge in the vena cava, in the right side of the heart, or in branches of the pulmonary artery (Figure 3-7). Adverse results include thrombosis, infection, and perforation. Although electrode fractures have become less common because of the development of new alloys, they are still a significant cause of pacing failure (Figure 3-8). The usual sites of fracture are near the pulse generator, at sharp bends in the wires, and at the point where the electrodes are inserted into the epicardium. Although most electrode fractures are easily detected on routine chest radiographs, some subtle fractures may be demonstrated only on oblique views or at fluoroscopy. Perforation of the myocardium by an intravenous electrode usually occurs at the time of insertion or during the first few days thereafter. Perforation should be suspected when the pacemaker fails to sense or elicit a ventricular response. Plain radiographs show the electrode tip lying outside the right ventricular cavity (Figure 3-9). Summary of Findings for Internal Devices In the lungs, thick mucus secreted by mucosa in the trachea and bronchi blocks the air passages. The thick mucus is the result of an imbalance of sodium and chloride production and reabsorption. These mucous plugs lead to focal areas of lung collapse. Recurrent pulmonary infections are common because bacteria that are normally carried away by mucosal secretions adhere to the sticky mucus produced in this condition. Because of the recurring nature of the disease, by age 10 years many children have widespread bronchiectasis with the formation of large cysts and abscesses. In the pancreas, blockage of the ducts by mucous plugs prevents pancreatic enzymes from entering the duodenum. This process impairs the digestion of fat, resulting in failure of the child to gain weight and the production of large, bulky, foul-smelling stools. In about 10% of newborns with cystic fibrosis, the thick mucus causes obstruction of the small bowel (meconium ileus) (Figure 3-10). Bowel perforation with subsequent fatal peritonitis may occur. Radiographically, cystic fibrosis causes generalized irregular thickening of linear markings throughout the lungs that, when combined with the almost invariable hyperinflation, produces an appearance similar to that of severe chronic lung disease in adults (Figure 3-11). Computed tomography (CT) screening to detect structural lung damage and assess disease progression is becoming more accepted by clinicians. There is a concern regarding the radiation dose if CT were used on a routine basis (yearly), especially because people with cystic fibrosis are now living into their 30s. The progressive underaeration of the lungs in hyaline membrane disease results from a lack of surfactant and immature lungs. Surfactant consists of a mixture of lipids, proteins, and carbohydrates that creates a high surface tension, requiring less force to inflate and maintain the alveoli. Normally the alveolar cell walls produce lipoprotein, which maintains the surface tension within the alveoli. This tension permits the alveoli to remain inflated so that atelectasis does not occur. The disease process results from surfactant deficiency caused by cell immaturity or birth trauma. In addition to pronounced underaeration, the radiographic hallmark of hyaline membrane disease is a finely granular appearance of the pulmonary parenchyma (Figure 3-12). A peripherally extending air bronchogram develops because the small airways dilate and stand out clearly against the atelectasis in the surrounding lung. Frontal radiographs of the lower neck show a characteristic smooth, fusiform, tapered narrowing (hourglass shape) of the subglottic airway caused by the edema (Figure 3-13A), which is unlike the broad shouldering normally seen (Figure 3-13B). On lateral projections of the neck using soft tissue techniques, a rounded thickening of the epiglottic shadow gives it the configuration and approximate size of an adult’s thumb (Figure 3-14), in contrast to the normal, narrow epiglottic shadow resembling an adult’s little finger. Alveolar, or air-space, pneumonia, exemplified by pneumococcal pneumonia, is produced by an organism that causes an inflammatory exudate that replaces air in the alveoli, so that the affected part of the lung is no longer air containing but rather appears solid, or radiopaque (Figure 3-15). The inflammation spreads from one alveolus to the next by way of communicating channels, and it may involve pulmonary segments or an entire lobe (lobar pneumonia). Radiographic Appearance: Consolidation of the lung parenchyma with little or no involvement of the airways produces the characteristic air bronchogram sign (Figure 3-16). The sharp contrast between air within the bronchial tree and the surrounding airless lung parenchyma permits the normally invisible bronchial air column to be seen radiographically. The appearance of an air bronchogram requires the presence of air within the bronchial tree, which suggests that the bronchus is not completely occluded at its origin. Presence of an air bronchogram excludes the diagnosis of a pleural or mediastinal lesion because there are no bronchi in these regions. Because air in the alveoli is replaced by an equal or almost equal quantity of inflammatory exudate and because the airways leading to the affected portions of the lung remain open, there is no evidence of volume loss in alveolar pneumonia. Radiographic Appearance: The small patches of consolidation may be seen radiographically as opacifications that are scattered throughout the lungs, but are separated by an abundance of air-containing lung tissue (Figure 3-17); air bronchogram is absent. If consolidation causes obstructed airways, atelectasis is evident. Interstitial pneumonia is most commonly produced by viral and mycoplasmal infections. In this type of pneumonia, the inflammatory process predominantly involves the walls and lining of the alveoli and the interstitial supporting structures of the lung, the alveoli septa. Radiographic Appearance: The interstitial dispersal of the infection produces a linear or reticular pattern (Figure 3-18). When seen on end, the thickened interstitium may appear as multiple small nodular densities. Left untreated, interstitial pneumonia may cause “honeycomb lung,” which is demonstrated on CT as cystlike spaces and dense fibrotic walls (Figure 3-19). Treatment: Extensive inflammation of the lung can cause a mixed pattern of alveolar, bronchial, and interstitial pneumonias, and this pattern appears as opacifications representing pulmonary consolidation. Treatment for these types of pneumonias usually includes regimented doses of an antibiotic to eradicate the cause. Rest, hydration, and deep-breathing techniques (supportive therapy) help in treating the infectious process. Radiographic Appearance: Both types of aspiration cause multiple alveolar densities, which may be distributed widely and diffusely throughout both lungs (Figure 3-20). Because the anatomic distribution of pulmonary changes is affected by gravity, the posterior segments of the upper and lower lobes are most commonly affected, especially in debilitated or bedridden patients. The earliest radiographic finding in lung abscess is a spherical density that characteristically has a dense center with a hazy, poorly defined periphery. If there is communication with the bronchial tree, the fluid contents of the cavity are partly replaced by air, producing a typical air-fluid level within the abscess (Figure 3-21). A cavitary lung abscess usually has a thickened wall with a shaggy, irregular inner margin. CT can assist in the diagnostic process to demonstrate an ill-defined outer wall and rule out empyema (Figure 3-22). Radiographic Appearance: There are four basic radiographic patterns of primary pulmonary tuberculosis, as follows: 1. The infiltrate may be seen as a lobar or segmental air-space consolidation that is usually homogeneous, dense, and well defined (Figure 3-23). The apical lordotic projection best demonstrates the apices without superimposition of bony structures (Figure 3-24).
Respiratory System
Physiology of the respiratory system
Internal devices
Central Venous Catheters
Transvenous Cardiac Pacemakers
Internal Device
Correct Placement*
Complications
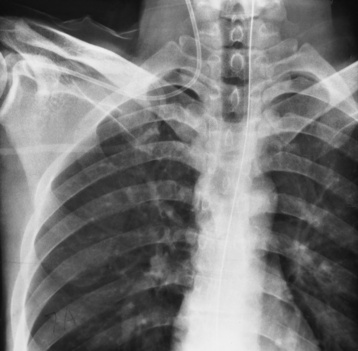
Endotracheal tube
Tip of tube 5-7 cm above the carina
Low placement—atelectasis
High placement—air entering the stomach
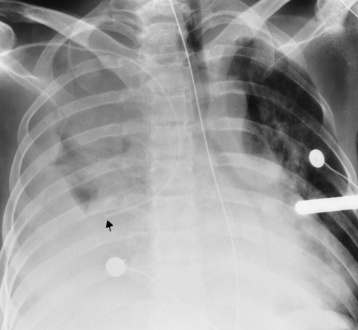
Central venous pressure catheters
Tip of catheter should be in the superior vena cava
Internal jugular vein placement
Right atrium—possible arrhythmias or perforation
Pneumothorax with placement
Infusion of fluid into mediastinum or pleural space
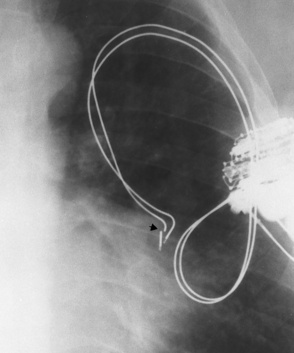
Swan-Ganz catheters
Right or left main pulmonary artery seen radiographically within the borders of the mediastinum
Pulmonary infarction
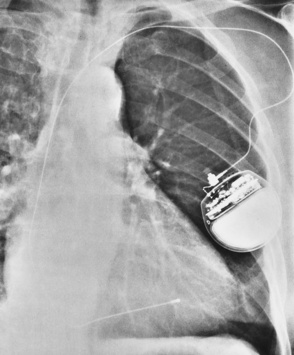
Transvenous cardiac pacemakers
Overexpose to demonstrate the tip of the electrode at the apex of the right ventricle
Coronary sinus placement—needs a lateral chest image to distinguish
Perforation at initial insertion
Congenital/hereditary diseases
Radiographic Appearance
Hyaline Membrane Disease
Radiographic Appearance
Inflammatory disorders of the upper respiratory system
Radiographic Appearance
Epiglottitis
Radiographic Appearance
Inflammatory disorders of the lower respiratory system
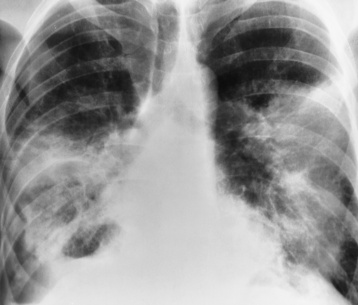
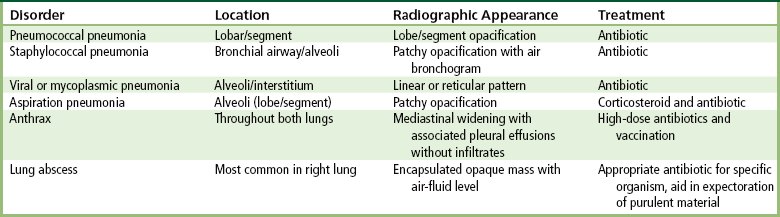
Alveolar Pneumonia
Bronchopneumonia
Interstitial Pneumonia
Aspiration Pneumonia
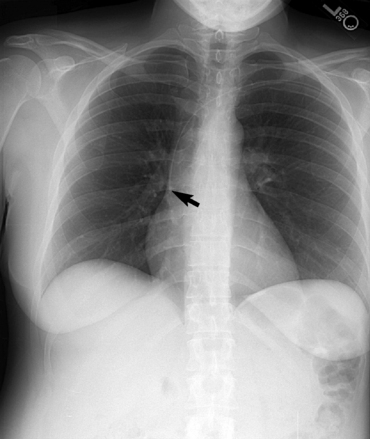
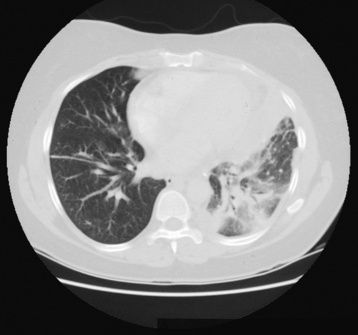
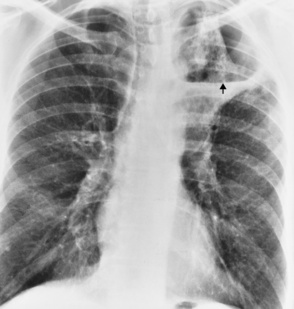
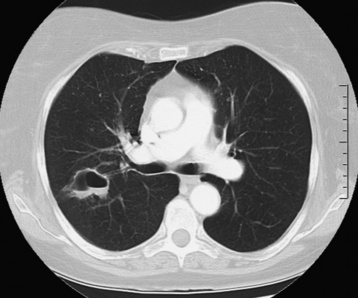
Lung Abscess
Radiographic Appearance
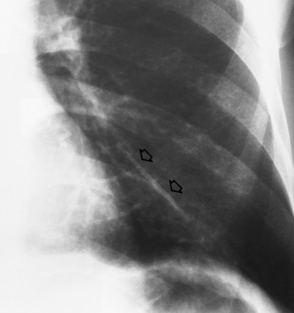
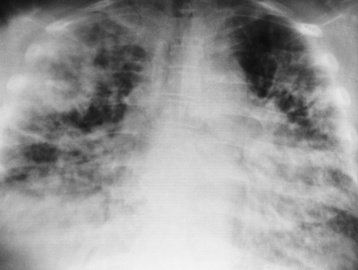
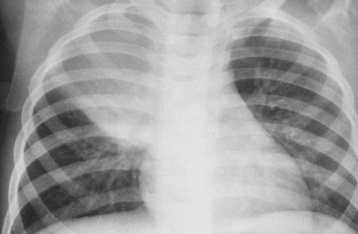
Tuberculosis
Primary Tuberculosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree