Introduction
Respiratory syncytial virus (RSV) is a common cause of lower respiratory infection and is highly contagious. By 2 years of age, it is estimated that all children will have been infected with this virus.1 Signs and symptoms vary; the majority of children present with an upper respiratory infection with rhinitis and cough. One-third of children also develop acute otitis media, and some may present with a low-grade fever. In the most severe cases, lower respiratory infection can cause bronchiolitis and/or pneumonia.1
Epidemiology
Annually in the United States, RSV accounts for approximately 58,000 hospitalizations and 2.1 million outpatient visits in young children less than 5 years of age.2 Infants less than 1 month old are at greatest risk for RSV hospitalizations.3 From 1976 to 1980, data from the Institute of Medicine estimated RSV deaths in children less than 5 years of age to be approximately 4,500 per year. However, current rates for children less than 2 years of age with a primary diagnosis of RSV are low—three to four deaths per 10,000 hospitalizations. Furthermore, almost all patients who died during a hospitalization associated with RSV infection had complex, chronic, underlying medical conditions.4
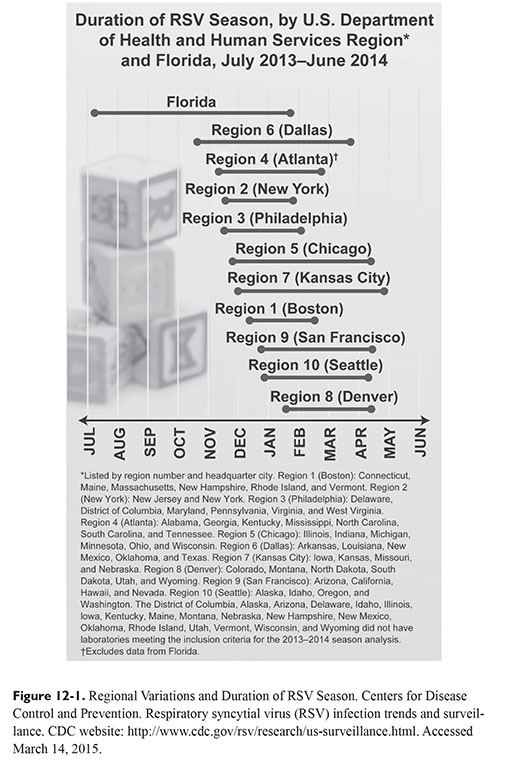
In the United States, RSV typically circulates during the fall, winter, and spring. Highest infection rates occur between December and March, but regional variations exist (Figure 12-1).5 Florida has an earlier seasonal onset of RSV with a longer duration.5,6 Urban areas also typically experience longer durations of the RSV season, which may be attributed to the increased spread of disease due to population size.7 To monitor onset, offset, peak, and duration of RSV, the Centers for Disease Control and Prevention monitors data voluntarily reported to the National Respiratory and Enteric Virus Surveillance System.8 Data are updated weekly, are publicly available, and can be used to determine the appropriate timing for RSV immunoprophylaxis.
Etiology and Pathogenesis
RSV is a single-stranded RNA-enveloped virus from the Paramyxovirus family and Pneumovirus genus. The viral genome encodes 10 viral proteins, including two glycoproteins that play a role in the pathogenesis of the virus. The F (fusion) and G (glyco) proteins are involved in the viral attachment of RSV to the host cells.9
The virus is spread through respiratory secretions with an incubation period of approximately 5 days.10,11 During early infection, viral replication occurs in the nasopharynx and then directly spreads to the respiratory epithelium.12,13 Bronchiolitis and/or pneumonia can occur as the virus spreads from the upper to the lower respiratory tract. During severe RSV infection, the necrosis of ciliated epithelial cells, infiltration of peribronchiolar mononuclear cells, and submucosal edema can lead to bronchiolar obstruction with patchy atelectasis.14–16 Infants with lower respiratory tract infections may present with wheezing, rales, and/or tachypnea 1 to 3 days after the onset of rhinorrhea.11,16 Bronchiolitis can lead to acute respiratory failure with hypoxia, hypercarbia, and severe bronchospasm. The most severe presentations typically occur in infants less than 2 months of age and in those born prematurely.1
RSV in Neonates
In the NICU, RSV can cause significant morbidity and mortality especially in premature neonates or infants with lung disease or congenital heart disease.17 Neonates with a low gestational age may be at higher risk for RSV infection because protection against the virus correlates with higher levels of maternal antibodies against RSV. With premature birth, transplacental transfer of protective maternal antibodies is interrupted, leaving neonates with an increased risk of acquiring infections.18,19
Because RSV transmission is usually seen with direct contact, healthcare workers or caregivers can bring the virus into neonatal units when it is typically circulating throughout the general population. Current recommendations emphasize hand hygiene; wearing of gowns, masks, and gloves; and isolation of any infected neonate. During an RSV outbreak in the NICU, some institutions have studied immunoprophylaxis with palivizumab, but due to the lack of data this practice is not supported by the 2014 American Academy of Pediatrics (AAP) guidelines.20–25
Prevention of RSV and Palivizumab
Palivizumab (Synagis®) was approved in 1998 for the prevention of serious lower respiratory tract disease caused by RSV in children at high risk.26 In the IMpact-RSV study, the clinical trial that led to palivizumab’s approval, the study showed a reduction in the incidence of RSV hospitalization from 10.6 to 4.8% (P <0.001) in those who received palivizumab. Children included in this study were less than or equal to 24 months of age with chronic lung disease and those less than or equal to 6 months of age and born preterm (at or before 35 weeks’ gestational age).27 In another trial conducted in children with hemodynamically significant congenital heart disease aged less than or equal to 24 months, palivizumab reduced the rate of RSV hospitalization from 9.7 to 5.3% (P = 0.003).28 To date, these two trials are the only randomized, placebo-controlled, double-blind studies that have been conducted to assess the safety and efficacy of palivizumab. Neither trial, nor any other trials that followed, has shown a statistically significant reduction in mortality from RSV with palivizumab.
Palivizumab provides passive immunity against RSV. It is a recombinant humanized monoclonal antibody that binds to the RSV F protein located on the envelope of the virus. The binding of the antibody to the F protein blocks viral fusion to host cells and blocks cell-to-cell fusion of RSV-infected cells.26 Although palivizumab may block cell-to-cell transmission of RSV, it is not indicated for the treatment of RSV and has not been shown to reduce the severity of the infection in children who have had breakthrough infection while receiving palivizumab prophylaxis.26
Palivizumab is a monthly 15 mg/kg intramuscular injection, typically administered in the thigh. It does not interfere with other live or inactivated vaccines, which allows childhood vaccines to be administered as scheduled. The first dose is administered prior to the start of the RSV season and monthly thereafter for a total maximum of five monthly doses per RSV season.21,26 In neonates born during the RSV season, palivizumab should be given only during the defined season and will require fewer than five doses.21 Palivizumab provides protection against the RSV virus for approximately 1 month; common side effects include fever and rash. After initial exposure or re-exposure, some children have experienced severe allergic reaction.26
Palivizumab Guidelines for Use
The AAP receives input from within and outside organizations such as the American College of Chest Physicians, American College of Emergency Physicians, American Thoracic Society, Emergency Nurses Association, National Association of Neonatal Nurses, National Association of Neonatal Nurse Practitioners, and Society of Hospital Medicine.21 Since palivizumab’s approval in 1998, the AAP has updated its guidelines four times—most recently in 2014. The current guidelines (after unanimous consensus from 21 committees, councils, sections, and advisory groups) define children as having the greatest risk of serious RSV disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree