Respiratory Pathology
ANATOMY
What is the primary respiratory muscle?
The diaphragm
What are the accessory muscles of respiration?
Intercostals, sternocleidomastoid, scalene, and abdominal muscles
What nerves innervate these muscles for effective ventilation?
Phrenic, intercostals, cranial, and cervical nerves
When are accessory muscles of respiration recruited for ventilation?
When there is a need to increase intrathoracic pressure to force exhalation, like in obstructive lung disease
HISTOLOGY
What types of cells line alveoli?
- Type I pneumocytes—predominant cell type that facilitate rapid diffusion of gases
- Type II pneumocytes—secrete surfactant (dipalmitoyl phosphatidylcholine)
What is unique about type II pneumocytes?
They are capable of regeneration and repair, and are precursors to type I pneumocytes.
What type of cell is a histiocyte?
A type of macrophage
What characteristic inclusion bodies can be found on electron microscopy in the cytoplasm of Langerhans histiocytes?
Birbeck granules (resemble tennis rackets)
PHYSIOLOGY
What is surfactant?
Dipalmitoyl phosphatidylcholine—a complex lipoprotein that coats the surface of alveoli, decreasing surface tension, and preventing collapse at low lung volumes
What increases production of surfactant?
Thyroxine and cortisol
What is residual volume (RV)?
The amount of air in the lungs after maximal expiration
What is expiratory reserve volume (ERV)?
The amount of air that can still be breathed out after normal expiration
What is alveolar volume (VA) and dead space volume (VD)?
- VA—the portion of an inhaled breath that fills the respiratory zone
- VD—the portion of an inhaled breath that remains in the conducting airways
What is tidal volume (TV or VT)
The sum of alveolar and dead space ventilation with quiet breathing
What is inspiratory reserve volume (IRV)?
The amount of air in excess of tidal volume that moves into the lungs on maximal inspiration
What is vital capacity?
The sum of tidal volume, inspiratory reserve volume, and expiratory reserve volume. Alternatively, it is the total volume of air that can be inhaled starting from the point of maximal expiration. Vital capacity is equal to total lung capacity minus residual volume.
What is the functional residual capacity (FRC?
The resting lung volume at the end of passive expiration which is determined by the opposing elastic forces of the chest wall (outward) and the lungs (inward).
How do you calculate the FRC?
FRC = RV + ERV
What is the total lung capacity (TLC?
The total amount of air that the lungs can contain (IRV + TV + ERV + RV)
What fraction of the TLC is the normal FRC?
Less than 50%
What is inspiratory capacity (IC)?
The volume of gas that can be taken into the lungs on a full inspiration starting from the functional residual capacity (IC = IRV + TV)
What is compliance?
The change in lung volume produced by a given change in intrapleural pressure (C = ΔV/ΔP)
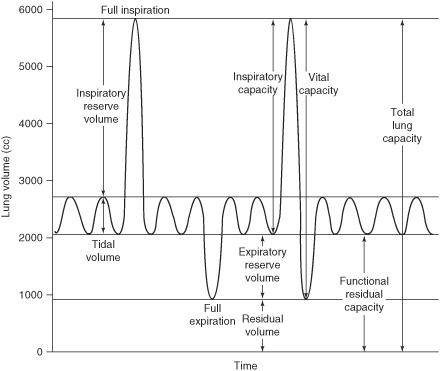
Figure 7.1 Lung volumes.
What conditions decrease compliance?
Restrictive lung diseases like pulmonary fibrosis or pulmonary edema which limit lung volume expansion
Give an example of a disease with increased lung compliance:
Emphysema increases compliance due to the loss of elastic recoil.
Describe the distribution of ventilation in the lungs:
Distribution is unequal, with greater ventilation at the apex and less at the base when in the upright position.
Describe the distribution of perfusion in the lungs:
Distribution is unequal, with greater perfusion at the base and less at the apex when in the upright position.
What kind of resistance circuit is the pulmonary circulation?
Low resistance
What determines blood flow in the normal lung?
The relationship between alveolar and pulmonary vascular pressure
What optimizes gas exchange?
The matching of ventilation and perfusion (V/Q)
What is the strongest factor affecting ventilation?
The maintenance of normal blood pH which is accomplished through the elimination or retention of CO2
What are the two types of respiratory sensors and where are they located?
- Chemoreceptors—found in the medulla and aortic and carotid bodies
- Mechanoreceptors (including stretch and irritant receptors)—found in the chest wall and airways
Chemoreceptors maintain normal blood pH by responding to changes sensed by central and peripheral receptors. What are these changes?
Central chemoreceptors monitor CSF and respond rapidly to changes in hydrogen ion concentration and pCO2. Peripheral chemoreceptors respond to changes in the partial pressure of arterial oxygen and exert regulatory effect by altering the respiratory rate.
How do chemoreceptors help maintain normal blood pH?
They modulate the rate and depth of breathing in response to how much the receptors are stimulated.
What is the difference between hypoxia and hypoxemia?
Hypoxia is a situation in which tissues are deprived of oxygen needs. Hypoxemia refers to decreased partial pressure of oxygen in the blood.
What happens to hemoglobin during normal conditions at 150 mm Hg PaO2?
Hemoglobin is completely saturated with four molecules of oxygen. Further increases in PaO2 have little effect on the oxygen content of blood.
PATHOLOGY
General Principles
What happens when alveolar pressure is greater than arterial pressure?
Perfusion is reduced or completely obstructed.
What two conditions can result in alveolar pressure being greater than arterial pressure?
- Shock—pulmonary artery pressure falls below alveolar pressure due to severe blood loss
- Positive pressure ventilation—alveolar pressure rises above the pulmonary artery pressure
What occurs in states of increased oxygen demand?
CO2 rises and pulmonary vascular resistance falls secondary to the recruitment of unperfused vessels in order to meet oxygen demand.
What happens to blood vessels in localized alveolar hypoxia?
There is local constriction of arterioles supplying the hypoxic area, also known as hypoxic pulmonary vasoconstriction.
How does hypoxic pulmonary vasoconstriction work?
Constriction of blood vessels decreases blood flow to areas of low ventilation and helps maintain ventilation-perfusion matching by directing blood to areas of higher ventilation.
What parameters influence the degree of oxygen saturation of hemoglobin?
The oxygen affinity for hemoglobin is regulated by [H+], [CO2], [2,3-BPG], temperature, and metabolic needs of the tissue. In peripheral tissues where there are conditions of increased acidity, increased [CO2], and increased [2,3-BPG], oxygen has a lower affinity for hemoglobin (right shift on the curve means that it takes higher pO 2 to saturate a given percentage of binding sites on hemoglobin). In the lungs where there are conditions of less acidity, decreased [CO2], and decreased [2,3-BPG], oxygen has a higher affinity for hemoglobin. This serves to facilitate oxygen unloading in peripheral tissues and oxygen binding in the lungs.
How does carbon monoxide (CO) affect the oxyhemoglobin dissociation curve?
CO binds to hemoglobin with 240 times the affinity of oxygen; consequently, it decreases the O2 content in blood by decreasing the amount of oxygen bound to hemoglobin. Thus for essentially any pO2, oxygen saturation of hemoglobin will be reduced if carbon monoxide is present.
What are the factors that affect pulmonary gas exchange?
Mismatching of ventilation with perfusion caused by hypoventilation, decreased FiO2, shunting, and diffusion impairment
Define shunting:
Deoxygenated blood passes through the pulmonary vasculature without being ventilated.
What are the nonpulmonary causes of hypoxemia?
Inadequate cardiac output, low hemoglobin concentration, and low hemoglobin-O2 saturation
How does aging affect normal lung function?
Both the total alveolar surface area and the elastic recoil of the lungs decrease
What is dyspnea?
Shortness of breath
What is orthopnea?
Dyspnea occurring when the patient is in the supine position as a result of a decrease in vital capacity caused by abdominal contents exerting force against the diaphragm
What is paroxysmal nocturnal dyspnea?
Dyspnea occurring several hours after lying down and is often associated with congestive heart failure. It is caused by an increase in venous return to the heart resulting in mild pulmonary edema.
What is atelectasis?
Alveolar collapse caused by bronchial obstruction or external compression of the lung parenchyma by tumors, pleural fluid, or air within the pleural cavity.
What is pulmonary alveolar proteinosis?
An uncommon condition characterized by the accumulation of amorphous, periodic acid-Schiff (PAS)-positive material in the alveolar air spaces.
What is a transudative pleural effusion?
Extravasated pleural fluid that occurs secondary to increased capillary pressure or low levels of serum protein
What is an exudative pleural effusion?
A collection of pleural fluid rich in protein and cellular elements that is caused by the altered permeability of vessel walls usually due to inflammation or malignancy
How do you differentiate between transudates and exudates in pleural fluid analysis?
By comparing protein and lactate dehydrogenase levels in the pleural fluid to the serum (Light criteria)
What are the Light criteria and how many must be met to diagnose an exudative pleural effusion?
At least one of the following criteria must be met:
- Pleural fluid protein >2.9 g/dL (29 g/L)
- Pleural fluid cholesterol >45 mg/dL (1.16 mmol/L)
- Pleural fluid LDH >60% of upper limit for serum
What are the principal causes of pleural exudates?
- Microbial infection
- Cancer including bronchogenic carcinoma, metastatic neoplasms, and mesothelioma
- Pulmonary infarction
- Viral pleuritis
Congenital
What are the most important factors for the survival of premature infants?
Adequate vascularization and surfactant in the lungs; surfactant generally begins to be produced at 32 weeks gestation.
What is pulmonary agenesis?
The complete absence of lungs, bronchi, and vasculature caused by failure of bronchial buds to develop
What is pulmonary hypoplasia?
Poorly developed bronchial tree with abnormal histology found in association with congenital diaphragmatic hernias and bilateral renal agenesis
Describe the association between pulmonary hypoplasia and congenital diaphragmatic hernia:
Herniation of abdominal contents into the thorax compresses the developing lung causing it to become hypoplastic.
What causes infant respiratory distress syndrome (hyaline membrane disease)?
Lack of or inadequate surfactant production plus structural immaturity
How do you measure lung maturity in a premature infant?
By measuring the lecithin-to-sphingomyelin ratio in the amniotic fluid. If the ratio is less than 2:1, the fetal lungs may be surfactant deficient.
What conditions are associated with infant respiratory distress syndrome (hyaline membrane disease)?
Prematurity, maternal diabetes mellitus, and birth by caesarean section
What is Kartagener syndrome?
An autosomal recessive disorder that results in structurally abnormal cilia leading to impaired mucociliary clearance in the airways and reduced sperm motility in the gonads
What cardiac anomaly is associated with Kartagener syndrome?
Situs inversus
What is Langerhans cell histiocytosis (histiocytosis X)?
A disease of the immune system in children that causes proliferation of histiocytes and may result in interstitial lung disease, painful bone swelling, and diabetes insipidus. The finding of diabetes insipidus, exophthalmos, and lytic bone lesions is also called Hand-Schüller-Christian triad. Langerhans cell histiocytosis (LCH) exists on a spectrum from unifocal disease (previously known as eosinophilic granuloma) to multifocal unisystem LCH (Hand-Schüller-Christian) to multifocal multisystem LCH (also called Letterer-Siwe disease).
Anatomic
What is a pneumothorax?
A collection of air or gas in the pleural cavity as a result of disease or injury
What is a hemothorax?
A collection of whole blood in the pleural cavity caused by the rupture of blood vessels resulting from trauma or inflammation
What are the causes of massive hemoptysis (greater than 500 cc of blood)?
Lung cancer, lung cavities containing mycetomas, cavitary tuberculosis, pulmonary hemorrhage syndromes, atrioventricular (AV) malformations, and bronchiectasis
How can you differentiate between a hemothorax and a bloody pleural effusion?
Blood clots are usually present in a hemothorax
What is a chylothorax?
A pleural collection of a milky lymphatic fluid containing microglobules of lipid
Why is a chylothorax always significant?
It implies obstruction of the major lymph ducts usually by an intrathoracic cancer.
Inflammatory/Autoimmune
What are the three most common causes of chronic cough?
- Asthma
- Postnasal drip
- Gastroesophageal reflux disease (GERD)
What are the two major categories of diffuse pulmonary lung disease?
- Obstructive
- Restrictive lung disease
What is the key feature in obstructive lung disease?
Increase in resistance of airflow out of the lungs due to the partial or complete obstruction of the airways resulting in lung volumes greater than normal (air trapping)
What is the key feature in restrictive lung disease?
Reduced expansion of lung parenchyma accompanied by a decrease in TLC resulting in smaller than normal lung volumes
How do lung volumes differ in obstructive and restrictive lung disorders?
Obstructive lung disease is characterized by a marked decreased in the 1 second forced expiratory volume (FEV1) and a normal or increased forced vital capacity (FVC) resulting in a decreased FEV1/FVC ratio. In restrictive lung disease, the FEV1 and FVC are both decreased proportionately, resulting in a normal FEV1/FVC ratio.
Give examples of obstructive pulmonary diseases:
Asthma, emphysema, chronic bronchitis, bronchiectasis, cystic fibrosis, bronchiolitis, tumors, and aspiration of foreign objects
Give examples of restrictive pulmonary diseases:
Adult respiratory distress syndrome (ARDS), pneumoconiosis, sarcoidosis, idiopathic pulmonary fibrosis, and chest wall/skeletal abnormalities
How are chronic restrictive pulmonary diseases categorized?
They are divided by lung response which includes alveolitis, interstitial inflammation, and diffuse fibrosis with or without granuloma formation.
What is asthma?
A condition characterized by episodic, reversible bronchospasm resulting from an exaggerated bronchoconstrictor response to a variety of stimuli
What are the clinical manifestations of asthma?
Bronchoconstriction, airway inflammation, edema, and mucus secretion
What is status asthmaticus?
Severe paroxysm that does not respond to therapy and would persist in the absence of intervention resulting in hypercapnia, acidosis, and severe hypoxia
What is emphysema?
A condition characterized by the permanent enlargement of the airspaces distal to the terminal bronchioles accompanied by destruction of alveolar walls
What are the three types of emphysema?
- Centrilobular
- Panacinar
- Paraseptal
What is the distinctive feature of centrilobular emphysema?
Distal alveoli are spared while the central or proximal parts of the acini formed by respiratory bronchioles are affected.
Where in the lungs are lesions of centrilobular emphysema more common and severe?
Upper lung lobes
What is panacinar emphysema?
Emphysema that results in uniformly enlarged acini from the level of the respiratory bronchiole to the terminal blind alveoli.
Where in the lungs are lesions of panacinar emphysema more common and severe?
Lower lung lobes
What is panacinar emphysema associated with?
Loss of elasticity and α1-antitrypsin deficiency
What is distinctive about paraseptal emphysema?
The proximal portion of the acinus is normal, while the distal part is predominantly involved.
Where in the lungs are lesions of paraseptal emphysema more common and severe?
Emphysema is more striking adjacent to the pleura, along the lobular connective tissue septa, and at the margins of the lobules.
What is the proposed mechanism to explain alveolar wall destruction and airspace enlargement in emphysema?
Excess protease or elastase activity unopposed by appropriate antiprotease regulation
What is α1-antitrypsin?
A glycoprotein which is a major inhibitor of serine protease activity, particularly elastase, which is secreted by neutrophils during inflammation
How does smoking contribute to emphysema?
Smoking favors the recruitment of leukocytes and the release of elastase.
What is the classic clinical presentation of emphysema?
Patients are usually dyspneic and have a barrel chest. Their breathing is marked by prolonged expiration, hyperventilation, and relatively normal gas values (pink puffers).
What is the classic clinical presentation of chronic bronchitis?
Patients are usually obese, have less prominent dyspnea, and have a decreased respiratory drive. They retain CO2 and tend to be hypoxic and cyanotic (blue bloaters).
What are the complications of chronic obstructive pulmonary disease (COPD)?
Chronic hypoxemia can lead to pulmonary vascular spasm, pulmonary hypertension, and cor pulmonale.
Define chronic bronchitis:
A persistent cough resulting in sputum production for more than 3 months for at least two consecutive years
What contributes to airflow obstruction in chronic bronchitis?
Inflammation, fibrosis with resultant narrowing of the bronchioles, and coexistent emphysema
What is the distinctive feature of chronic bronchitis?
Hypersecretion of mucus
What is chronic bronchiolitis?
Small airway disease characterized by goblet cell metaplasia, inflammation, fibrosis, and smooth muscle hyperplasia
What is bronchiectasis?
The permanent dilation of bronchi and bronchioles due to the destruction of muscle and elastic tissue secondary to infection or obstruction caused by a variety of conditions
Patients with bronchiectasis classically complain of what symptom complex?
Cough with copious amounts of purulent, sometimes fetid, sputum
What are the conditions that commonly predispose to bronchiectasis?
- Bronchial obstruction caused by tumors, foreign bodies, and mucus impaction
- Congenital or hereditary conditions like cystic fibrosis, immunodeficiency states, and Kartagener syndrome
- Necrotizing or suppurative pneumonia
What is usually cultured from the sputum of patients with bronchiectasis?
Mixed flora including staphylococci, streptococci, pneumococci, enteric organisms, anaerobic and microaerophilic bacteria, Haemophilus influenzae, and Pseudomonas aeruginosa.
What causes restrictive lung disease?
Abnormalities of the chest wall due to bony deformities or neuromuscular dysfunction; interstitial lung disease—characterized by accumulation of substances within the pulmonary interstitium
What are the key changes that occur in restrictive lung disease?
Interstitial fibrosis produces a stiff lung with reduced lung compliance necessitating increased respiratory effort.
What are the complications of restrictive lung disease?
Respiratory failure, pulmonary hypertension, and cor pulmonale
What are the prototypic acute restrictive (interstitial) lung disorders?
Acute respiratory distress syndrome (ARDS) and infant respiratory distress syndrome (hyaline membrane disease)
What is acute respiratory distress syndrome (ARDS)?
A syndrome featuring acute respiratory compromise in the absence of left-sided heart failure resulting from diffuse alveolar damage and an increase in capillary permeability causing leakage of protein-rich fluid into the alveoli. This syndrome can be the result of many different etiologies.
What is the mechanism of injury in ARDS?
Necrosis of endothelial and epithelial cells secondary to the release of toxic mediators by neutrophils, the formation of oxygen-derived free radicals, and the activation of the coagulation cascade
What is the classic radiographic finding in ARDS?
Diffuse ground-glass opacification in the lungs
What is the characteristic pathologic finding in ARDS?
Intra-alveolar hyaline membranes composed of fibrin and cellular debris
What is idiopathic pulmonary fibrosis (IPF)?
An interstitial lung disease of unknown etiology that is characterized by chronic inflammation and fibrosis of the alveolar wall
Describe the sequence of events in IPF:
It begins with alveolitis, progresses to fibrosis, and results in a lung filled with cystic spaces (honeycomb lung)
What is observed clinically in a patient with IPF?
Patients exhibit respiratory difficulty and eventually become hypoxemic and cyanotic. Cor pulmonale and cardiac failure may result.
What is hypersensitivity pneumonitis (extrinsic allergic alveolitis)?
An immunologically mediated inflammatory lung disease that results in alveolitis. It is often an occupational disease that results from heightened sensitivity to inhaled antigens.
How does hypersensitivity pneumonitis usually present?
The acute reaction presents with fever, cough, dyspnea, and constitutional complaints 4 to 8 hours after exposure. The chronic form of the disease has an insidious onset of cough, dyspnea, malaise, and weight loss.
How does hypersensitivity pneumonitis differ from bronchial asthma?
In bronchial asthma, the bronchi are the focus of injury; whereas in hypersensitivity pneumonitis, damage occurs at the level of the alveoli and results in a restrictive picture.
What are diffuse pulmonary hemorrhage syndromes?
Pulmonary interstitial and vascular disorders that present with hemorrhage. They include Goodpasture syndrome, idiopathic pulmonary hemosiderosis, and vasculitis-associated hemorrhage.
What is sarcoidosis?
A type IV hypersensitivity reaction to an unknown antigen that results in a multisystem disease characterized by noncaseating granulomas in multiple tissues and organs.
Sarcoidosis tends to affect what race and age group?
People of African descent during the teenage or young adult years
What is the most common abnormality seen on routine x-ray in a patient with sarcoidosis?
Bilateral hilar lymphadenopathy
What are the characteristic laboratory findings in sarcoidosis?
Hypercalcemia/hypercalciuria, hypergammaglobulinemia, and increased activity of serum angiotensin-converting enzyme (ACE)
What are the common pathologic changes that occur in sarcoidosis?
Interstitial lung disease; enlarged hilar lymph nodes; anterior uveitis; splenomegaly/hepatomegaly; erythema nodosum of the skin; polyarthritis
What is Mikulicz syndrome?
Bilateral eye and salivary gland involvement in sarcoidosis, tuberculosis, or leukemia
What sort of immunologic response is seen in patients with sarcoidosis?
Patients manifest cutaneous allergy to common skin test antigens like Candida, mumps, and purified protein derivative (PPD). They also have a polyclonal hyperglobulinemia.
What is the clinical course of sarcoidosis?
It is largely unpredictable and characterized by either progressive chronicity or periods of activity interspersed with remissions.
It requires lung or lymph node biopsy demonstrating noncaseating granulomas.
What is Goodpasture syndrome?
A hemorrhagic pneumonitis and glomerulonephritis caused by antibodies to antigens common to glomerular and pulmonary basement membranes
What is idiopathic pulmonary hemosiderosis?
A disease that resembles the pulmonary component of Goodpasture syndrome without the renal component
Environmental/Toxins
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


