Respiratory Distress Syndrome
Introduction
Have you ever blown up a balloon? The first couple of breaths are especially challenging because the insides of new balloons are often sticky. In newborn lungs, the alveoli are similar to new balloons. In term, healthy infants, the alveoli are coated by pulmonary surfactant that reduces surface tension and allows the alveoli to expand as air flows into the lungs. The primary cause of respiratory distress syndrome (RDS) is inadequate pulmonary surfactant that leads to diffuse alveolar atelectasis, edema, and cell injury. The production of pulmonary surfactant begins at 22 weeks’ gestation, and the fetal lungs mature at 35 weeks. Prematurity is the most common cause of RDS. Despite the improvement in perinatal and neonatal care, RDS remains a significant cause of morbidity and mortality in premature infants and especially the extremely premature group.1,2
Epidemiology and Risk Factors of RDS
Approximately 10% of premature infants in the United States develop RDS every year.3 The highest incidence occurs in the most premature infants. In infants born before 29 weeks’ gestation, the chance of developing RDS is 60%.4 Maternal risk factors include poor prenatal care, being uninsured, poverty, being a member of a minority group, history of a preterm birth, low maternal body mass, and periodontal disease. Maternal diabetes affects lung development and increases the risk of RDS.5 Some thoracic malformations, such as congenital diaphragmatic hernia, cause lung hypoplasia and can increase the risk of RDS.
Prematurity is the most significant risk factor for RDS. Perinatal asphyxia may acutely impair surfactant production, increasing the risk of RDS. During the labor process, adrenergic and steroid hormone release promotes surfactant release. Cesarean section without labor, therefore, can increase the risk of RDS in term and late preterm infants. Among preterm infants, the RDS risk also increases with male gender and Caucasian race.
Very rarely, genetic disorders of surfactant production and metabolism cause RDS-like symptoms in term infants. Examples of these disorders include surfactant protein B and surfactant protein C mutations as well as mutations of the ABCA3 gene, which affects alveolar cells. These disorders are usually fatal without lung transplantation.6
Pathophysiology of RDS
Surfactant Deficiency
The primary cause of RDS is pulmonary surfactant deficiency. Surfactant is essential for alveoli to overcome surface tension and stay open, allowing gas exchange to occur. Lacking surfactant and being structurally immature, the premature neonatal lung has decreased compliance and a higher risk of atelectasis.6
Hypoxemia, Hypercarbia, and Lactic Acidosis
The ventilation/perfusion (V/Q) mismatch seen with atelectasis is a result of well perfused but poorly ventilated areas of the lung. V/Q mismatch causes hypoxemia and hypercarbia. Severe hypoxemia and systemic hypoperfusion lead to decreased oxygen delivery, anaerobic metabolism, and subsequent respiratory and metabolic lactic acidosis.
Pulmonary Vasoconstriction
Hypoxemia and lactic acidosis may cause pulmonary vasoconstriction and right-to-left shunting through the foramen ovale and ductus arteriosus, further impairing oxygenation.
Oxygen Damage, Barotrauma, and Volutrauma
With atelectasis, pressure exerted trying to open the alveoli can rupture the alveoli causing further damage to the lung tissue. A high fraction of inspired oxygen may also injure the lung tissue because oxygen is a free radical. These damages may initiate release of inflammatory cytokines and chemokines, worsening the endothelial and epithelial cell injury. The injury reduces surfactant synthesis and increases endothelial permeability, resulting in pulmonary edema. The increased endothelial permeability also allows leakage of protein into the alveolar space, inactivating surfactant and further exacerbating surfactant deficiency.
Clinical Presentation of RDS
A premature infant with RDS shows clinical signs soon after birth. The classic signs include tachypnea, intracostal and subcostal retractions, nasal flaring, grunting, and cyanosis in room air.7
Management of RDS
The goals of RDS management are
 avoiding hypoxemia and acidosis
avoiding hypoxemia and acidosis
 minimizing lung injury from volutrauma and oxygen toxicity
minimizing lung injury from volutrauma and oxygen toxicity
 optimizing fluid management—to prevent hypovolemia and hypotension without overloading with fluid
optimizing fluid management—to prevent hypovolemia and hypotension without overloading with fluid
 reducing metabolic demands and maximizing nutrition
reducing metabolic demands and maximizing nutrition
Some important advances in preventing and treating RDS are antenatal corticosteroids, surfactant replacement therapy, continuous positive airway pressure, and positive end-expiratory pressure. This chapter will focus on the pharmacologic interventions.
Antenatal Corticosteroids
Antenatal corticosteroids accelerate maturation of the fetal lung and other fetal tissues, reducing the risk of RDS, intraventricular hemorrhage, and necrotizing enterocolitis. Corticosteroids also induce surfactant production. Antenatal corticosteroids should be given to pregnant women at 24 to 36 weeks’ gestation who are at risk for preterm delivery within 7 days. Antenatal corticosteroids can be given to pregnant women with intact or ruptured membranes as long as they do not have chorioamnionitis, which is a contraindication to antenatal corticosteroids. A full course of antenatal corticosteroids consists of betamethasone 12 mg via intramuscular (IM) injection every 24 hours for two doses or dexamethasone 6 mg IM every 12 hours for four doses.7 Women in preterm labor may not always be able to complete the full treatment course, but an incomplete course may still be beneficial.7 The efficacy of antenatal corticosteroids at gestational age under 24 weeks is unknown, but antenatal corticosteroids in this population may still be justified if the therapy benefits are believed to outweigh risks.
Surfactant Replacement Therapy
Surfactant replacement therapy was a significant advancement in the care of infants with RDS. Since the introduction of surfactant replacement therapy, the mortality rate from RDS has significantly declined from about 25,000 deaths each year in the 1960s to 860 deaths in 2005.8 Surfactant replacement therapy is one of the most well-studied drug therapies in neonates and has consistently been shown to benefit infants with RDS. Studies with surfactant replacement therapy generally show improved oxygenation and decreased ventilation support. Many studies also showed decreased incidence of air leak syndrome and death.
Surfactant can be given to infants with high risk for RDS soon after birth prophylactically before lung injury occurs. Compared to “rescue” surfactant, prophylactic surfactant improves air distribution and reduces lung injury. An “early rescue” treatment given within 2 hours of birth is also proven to be beneficial over delayed treatment. The optimal administration time for the first dose of replacement surfactant, whether it is at birth or within 2 hours after birth, remains to be determined.
Infants with established RDS have been shown to benefit from a repeated dose of surfactant. These benefits include improvement in oxygenation and ventilation, decreased risk for pneumothorax, and increased survival compared to infants who only received one dose of surfactant. No additional benefit, however, has been observed beyond three doses of poractant alfa, four doses of calfactant, or four doses of beractant. Criteria for retreatment vary among neonatologists because there is no established guideline on when and to whom retreatment should be given. Very often, the decision to repeat surfactant therapy is made based on facility-specific criteria or the patient’s clinical status.
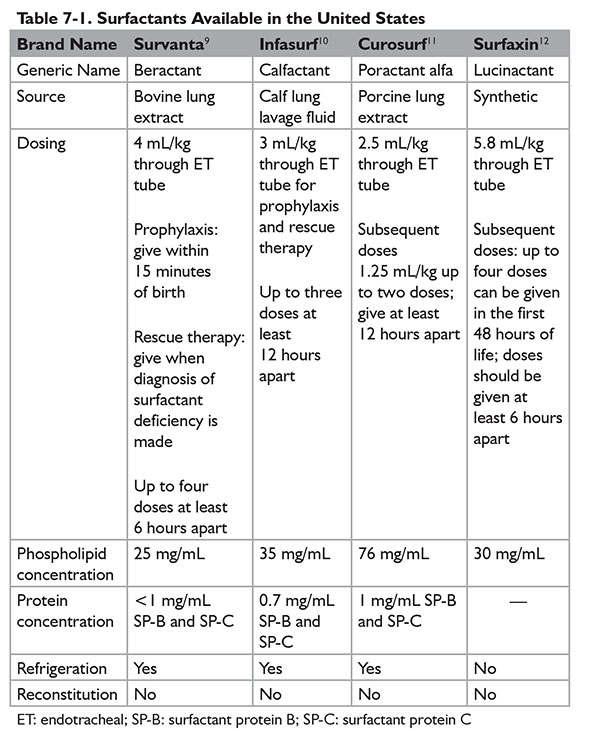
Surfactant is administered via an endotracheal tube. Administration of surfactant via nebulization has not been successful but continues to be evaluated by ongoing trials. Currently, four products under the brand names of Survanta, Infasurf, Curosurf, and Surfaxin are available in the U.S. market. (Refer to Table 7-1 for further dosing information.) During the administration of surfactant, ventilation needs to be briefly disconnected. The most common adverse effects are apnea, bradycardia, and desaturation. Pulmonary hemorrhage, which happens more frequently in extremely low birth weight infants, is a rare but serious adverse effect of surfactants. Other risk factors for developing pulmonary hemorrhage with surfactant therapy include male gender and patent ductus arteriosus.
Long-Term Complications of RDS
Long-term complications of RDS include bronchopulmonary dysplasia (BPD), neurodevelopmental impairment, retinopathy of prematurity, and other complications of prematurity. The risk of developing these complications is inversely proportional to the infant’s gestational age.
Conclusion of RDS
To summarize, RDS is a significant cause of morbidity and mortality in premature infants. It is primarily caused by surfactant deficiency. Prematurity is the most significant risk factor for RDS. To reduce the risk of RDS, antenatal corticosteroids should be initiated in women at risk for preterm labor because they can accelerate lung maturation. The goals of RDS management are avoiding hypoxemia, minimizing lung injury, and optimizing fluid management and nutritional support. Surfactant replacement therapy has well documented benefits in neonates with RDS and should be given soon after birth in neonates with high risk for RDS. The numerous long-term complications of RDS include BPD.
Bronchopulmonary Dysplasia
Introduction
BPD, also known as chronic lung disease of prematurity, is a long-term complication of RDS and the most common chronic lung disease of childhood. BPD is a result of the oxygen and mechanical ventilation required in managing severe RDS in premature infants.13 It is an important cause of respiratory illness in preterm infants and has a high morbidity and mortality rate.
Definition of BPD
The presence and severity of BPD is defined by a set of criteria released by the National Institute of Child Health and Human Development (NICHHD).14,15 According to the NICHHD, an infant has BPD if he or she has received treatment with oxygen >21% for at least 28 days. The NICHHD further stratifies infants with BPD into different risk categories based on their birth gestational age and oxygen requirement at the time of assessment (see Table 7-2). These diagnosing criteria help predict the likelihood of BPD survivors requiring pulmonary medication and rehospitalization for pulmonary disease. Furthermore, per the NICHHD criteria, infants with severe BPD also have a higher risk for neurodevelopmental impairment.